Japanese Kobayashi Pharmaceutical's 'Hongguk' Health Supplement Scandal
120 Deaths Accumulated Over 3 Years... Cause 'Puberulin Acid'
Delayed Response Under Fire... CEO and Executives Resign Together in July
In Japan, it was revealed that 120 people died after taking a health supplement containing the 'Hongkuk (red yeast)' ingredient from Kobayashi Pharmaceutical, causing shock. An investigation found that the harmful substance was puberulic acid derived from blue mold. On the 18th, the Asahi Shimbun, citing Japan's Ministry of Health, Labour and Welfare, reported, "It is certain that puberulic acid derived from blue mold caused kidney damage in the victims who consumed the product."
 Akihiro Kobayashi, President of Kobayashi Pharmaceutical in Japan, held a press conference in Osaka on March 29, bowing his head in apology along with other executives. [Image source=Yonhap News]
Akihiro Kobayashi, President of Kobayashi Pharmaceutical in Japan, held a press conference in Osaka on March 29, bowing his head in apology along with other executives. [Image source=Yonhap News]
Japan's National Institute of Health Sciences has been investigating the relationship between the unintentionally contained ingredient in Kobayashi Pharmaceutical's product 'Hongkuk Cholesterol Help' and health damage through animal experiments and other methods. However, two other compounds found in the problematic product besides puberulic acid were reported to have no confirmed kidney toxicity. Although puberulic acid was previously detected in the product, it had not been confirmed as the causative agent until now. The Ministry of Health, Labour and Welfare estimated that blue mold contamination during the manufacturing process led to the production of toxic puberulic acid.
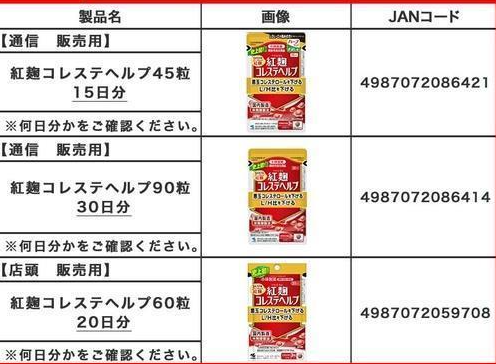 Products for which Kobayashi Pharmaceutical has requested discontinuation. [Image source=Captured from Kobayashi Pharmaceutical official website]
Products for which Kobayashi Pharmaceutical has requested discontinuation. [Image source=Captured from Kobayashi Pharmaceutical official website]
Hongkuk is made by fermenting rice and other grains with red yeast mold (Hongkuk-gyun), turning them red, and is known to have cholesterol-lowering effects. Approximately 1.1 million units of the product were sold since its release in 2021. However, over the past three years, there have been cases of death due to worsening kidney disease among those who consumed the product. According to the damage report submitted by Kobayashi Pharmaceutical on the 15th, 120 people died after taking the product. The company was criticized for its delayed response, as it only announced the issue in March despite being aware of the damage cases since January of this year.
In response, in July, Kazumasa Kobayashi, chairman of Kobayashi Pharmaceutical, and his son Akihiro Kobayashi, president, resigned together, taking responsibility for the incident. Chairman Kobayashi stepped down to a special advisory role, and his son stepped down as an unregistered executive to handle compensation and follow-up measures.
The company has also exported both the finished health supplement containing Hongkuk and the Hongkuk raw materials overseas. However, in March, the Korea Food and Drug Administration stated, "The problematic red yeast health foods (five types) are currently not officially imported into the country," and added, "To prevent direct overseas purchases of these products, detailed information about the products has been provided to domestic platform companies, and sales restraint has been requested."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















