A technology capable of removing tritium generated from nuclear power plants (hereinafter referred to as nuclear power plants) using a catalyst has been developed domestically.
Tritium is a representative radioactive substance generated when operating heavy water reactors, and it became widely known to the public last year when Japan discharged contaminated water from the Fukushima nuclear power plant into the ocean.
Tritium can adversely affect marine ecosystems and the environment, necessitating removal facilities. However, the technology developed by the research team is significant in that it can drastically remove tritium using a catalyst.
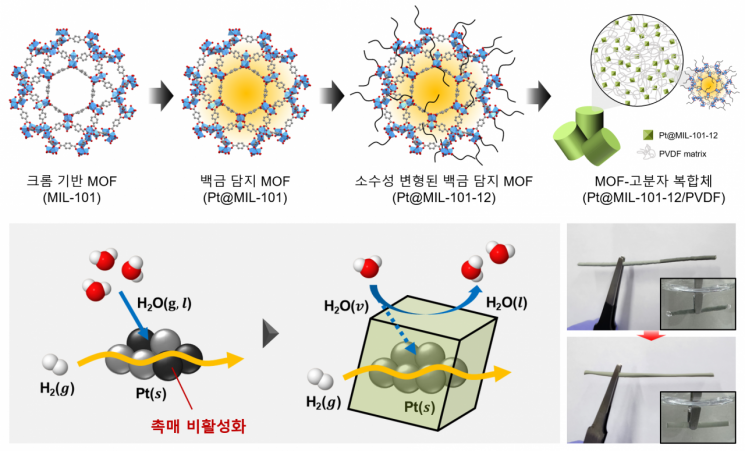 Hydrophobic modification of chrome-based metal-organic frameworks (MOFs) and the catalytic fabrication process of MOF-polymer composite structures. The catalyst developed by the research team prevents catalyst deactivation caused by direct contact with water and controls the ingress and egress of moisture at the molecular level. Provided by KAIST
Hydrophobic modification of chrome-based metal-organic frameworks (MOFs) and the catalytic fabrication process of MOF-polymer composite structures. The catalyst developed by the research team prevents catalyst deactivation caused by direct contact with water and controls the ingress and egress of moisture at the molecular level. Provided by KAIST
KAIST announced on the 27th that a research team led by Professor Ko Dong-yeon of the Department of Bio and Chemical Engineering at KAIST, in collaboration with Dr. Park Chan-woo’s team at the Korea Atomic Energy Research Institute, developed a new structure of a “dual-function (which blocks liquid water but allows gaseous steam to pass through) hydrophobic catalyst” necessary for the process that can remove tritium contained in nuclear power plant wastewater.
The catalyst developed by the joint research team showed a maximum reaction efficiency of 76.3% under specific reaction conditions. In particular, they concretely confirmed catalytic activity for low-concentration isotopes at the level of several hundred ppm, which had not been previously revealed.
Until now, liquid-phase catalytic exchange processes have mainly been used to remove tritium.
However, in this process, hydrogen-water isotope exchange reactions occur, and platinum, which is mainly used as a catalyst, has high reactivity but requires a high cost.
Therefore, evenly dispersing a small amount of platinum and introducing hydrophobic materials that repel water to activate the catalyst by moisture became the core of the process.
To overcome these problems of the existing process, the joint research team developed a new structure of tritium removal catalyst in the form of a composite of metal-organic frameworks and porous polymers.
This structure evenly distributes platinum particles with an average diameter of 2.5 nanometers (nm) on the metal-organic framework and imparts hydrophobicity through chemical modification.
This technology controls hydrophobicity at the molecular level to prevent the catalyst from losing activity due to water while allowing the necessary amount of water molecules for the reaction to easily access the catalyst.
 (From left) Heo Heeryeong, KAIST PhD candidate; Ko Dongyeon, KAIST professor; Park Chanwoo, Korea Atomic Energy Research Institute PhD; So Jungsup, Korea Atomic Energy Research Institute PhD. Provided by KAIST
(From left) Heo Heeryeong, KAIST PhD candidate; Ko Dongyeon, KAIST professor; Park Chanwoo, Korea Atomic Energy Research Institute PhD; So Jungsup, Korea Atomic Energy Research Institute PhD. Provided by KAIST
The joint research team confirmed that the developed catalyst exhibits excellent activity for tritium removal reactions even at very low isotope concentrations similar to nuclear power plant operating conditions. They also succeeded in demonstrating durability by maintaining a certain level of performance during continuous operation for four weeks.
Furthermore, the joint research team used in situ attenuated total reflectance infrared spectroscopy to observe the real-time movement of water molecules at the micro-molecular level. Through this, they proved that the catalyst suppresses deactivation caused by moisture while water molecules continuously approach the catalyst’s active sites to react.
In situ attenuated total reflectance infrared spectroscopy is a technique that analyzes information from light reflected off a material in real time to identify changes in the composition of the target material.
This study is significant in that it proposed a new structure of catalyst that can be used for tritium removal reactions by enhancing moisture resistance, the main cause of catalyst deactivation, through relatively simple hydrophobic control of metal-organic framework materials.
Professor Ko of KAIST explained, “The catalyst developed by the joint research team is expected to be applicable not only for tritium wastewater treatment but also as a core material for hydrogen isotope separation essential for technologies such as heavy water raw material production used in semiconductors and nuclear fusion fuel cycle technology.”
Meanwhile, this research was conducted with the support of the Korea Research Foundation’s core technology development project for enhancing nuclear power plant decommissioning safety. The first author of the paper is Heo Hee-ryeong, a doctoral student in the Department of Bio and Chemical Engineering at KAIST, and the research results were published on July 31 in the international environmental journal Energy and Environmental Materials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















