An optimized animal model for developing treatments for fatty liver disease has been developed in South Korea.
On the 19th, KAIST announced that a joint research team consisting of Professor Kim Ha-il’s team from the Graduate School of Medical Science and Engineering at KAIST, Professor Park Jun-yong’s team from Yonsei University College of Medicine, Hanmi Pharmaceutical R&D Center, and JD Bioscience Co., Ltd. developed a new animal model for metabolic abnormal fatty liver disease.
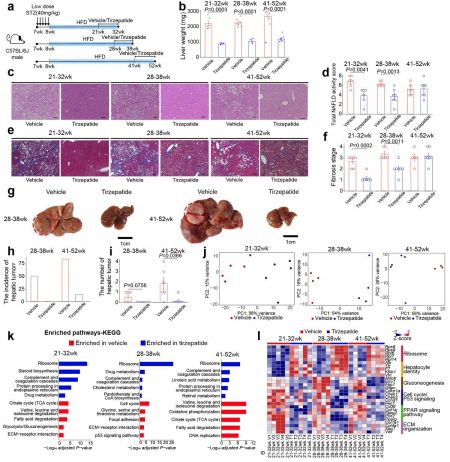 Visualization of the improvement effects of GLP-1 analogs on metabolic abnormality steatohepatitis, liver fibrosis, and liver tumor development. Provided by KAIST
Visualization of the improvement effects of GLP-1 analogs on metabolic abnormality steatohepatitis, liver fibrosis, and liver tumor development. Provided by KAIST
Metabolic abnormal fatty liver disease is a chronic condition that begins with fatty liver and progresses to steatohepatitis, fibrosis, cirrhosis, and liver cancer. Early appropriate treatment is crucial as mortality can increase due to cardiovascular diseases and liver-related complications.
The prevalence of this disease reaches 20-30%, and steatohepatitis affects more than 5% of the global adult population.
However, there are currently no commercialized treatments available. The lack of suitable animal models that can mimic human disease has hindered the elucidation of pathogenic mechanisms and the development of therapies.
In particular, existing animal models have failed to reflect whether metabolic abnormalities such as diabetes and obesity induce the onset of cirrhosis and liver cancer.
This is where the significance of the new animal model developed by the joint research team lies.
The joint research team focused on the fact that the prevalence of metabolic abnormal fatty liver disease accompanied by obesity and diabetes is higher in Asians with beta-cell dysfunction. They developed an animal model by injecting a drug into mice to destroy beta cells and induce diabetes, then feeding them a high-fat diet to rapidly progress fatty liver disease accompanied by obesity and diabetes.
In experiments conducted over one year using this mouse model, gradual development of fatty liver, steatohepatitis, liver fibrosis (hardening of parts of the liver), and liver cancer was observed. Genomic analysis of the mouse liver confirmed a state very similar to that of patients with metabolic abnormal fatty liver disease accompanied by obesity and type 2 diabetes.
The liver cancer that developed in the mouse model showed histological and molecular biological characteristics similar to those seen in liver cancer patients with metabolic abnormal fatty liver disease.
The joint research team also tested the effects of GLP-1 analogs, obesity treatments, on the developed animal model. During this process, administration of GLP-1 analogs inhibited the progression of fatty liver, hepatitis, and liver fibrosis in the mouse model, opening the possibility that this mouse model can be usefully applied as a preclinical model for new drug development.
Most importantly, the joint research team was the first to demonstrate that administration of GLP-1 analogs suppresses the occurrence of liver cancer, proposing the use of GLP-1 analogs to inhibit liver cancer development, a major cause of death in metabolic abnormal fatty liver disease.
 Professor Kim Ha-il. Provided by KAIST
Professor Kim Ha-il. Provided by KAIST
Professor Kim said, “Previously used animal models for metabolic abnormal fatty liver disease showed problems in properly reflecting the broad spectrum of the disease and metabolic disorders such as diabetes and obesity. However, the mouse model developed by the joint research team mimics the characteristics of chronic metabolic diseases and is expected to provide a turning point in research on animal models of metabolic abnormal fatty liver disease.”
Meanwhile, this research was conducted with support from the Ministry of Science and ICT, Ministry of Health and Welfare, Ministry of Education, and JD Bioscience. The research paper, completed with the participation of Dr. Jung Byung-kwan and Professor Choi Won-il from KAIST Graduate School of Medical Science and Engineering, and Professor Choi Won-seok from Hwasun Jeonnam National University Hospital as co-first authors, was published in the August 2 issue of the international journal Nature Communications.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.















