Celltrion's new autoimmune disease treatment drug, Jimpentra (marketed as Remsima SC in the US), is making strides toward becoming a global blockbuster therapy by being listed in 26 formularies managed by Prescription Benefit Managers (PBMs) and insurers, which serve as the gateway to the US market.
According to Celltrion on the 9th, Jimpentra achieved a significant milestone by signing formulary listing contracts with all three major US PBMs just five months after its US launch in March. After securing a listing contract with Express Scripts (ESI) within about two weeks of launch, the company successfully contracted with the remaining two PBMs on March 30 and April 2, respectively.
Jimpentra is accelerating its US market penetration by leveraging its convenience as the "world's only infliximab subcutaneous injection." The active ingredient, infliximab, has long been proven effective and safe for inflammatory bowel diseases such as ulcerative colitis and Crohn's disease. However, existing drugs containing this ingredient have only been available as intravenous injections administered at hospitals or clinics. Given the difficulty of visiting medical institutions in the US, there has been strong market demand for a subcutaneous injection formulation that patients can self-administer.
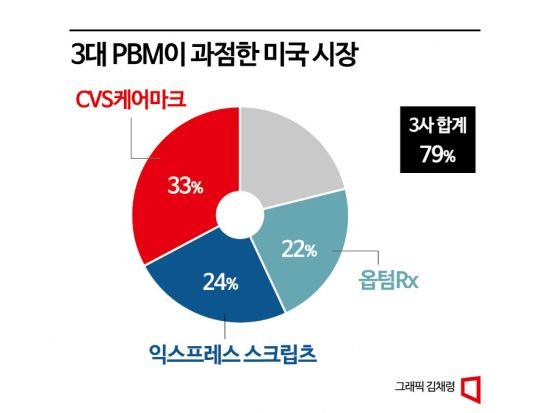
The key gateway to the US market is the PBM. Drugs sold in the US must be listed on formularies managed by PBMs on behalf of public and private insurers; otherwise, practical sales are difficult. If Jimpentra is not included in the formulary of the patient's insurance plan, the patient must bear the full cost of the drug, making it difficult for doctors to prescribe it despite its efficacy due to cost concerns. Currently, the US PBM market is dominated by three major PBMs?Express Scripts, CVS Caremark, and OptumRx?collectively holding about 80% market share.
Celltrion, however, has succeeded in listing Jimpentra on a total of 26 formularies, including the three major PBMs as well as small and medium-sized PBMs and insurers. This is estimated to secure approximately 75% coverage of the US insurance market. Even though all three major PBMs have been secured, coverage does not exceed 80% because formularies are further divided between public and private insurance. The large PBMs contracted by Celltrion on April 2 currently list Jimpentra only on public insurance formularies.
A Celltrion representative stated, "Only private insurance contracts through additional negotiations remain," adding, "As inquiries about Jimpentra continue from various regional insurers, it is expected that most of the US insurance market coverage will be secured by the end of this year." They emphasized that many regional insurers who have listed Jimpentra on their formularies did so independently without separate rebate negotiations, making profitability easier to secure.
Celltrion plans to secure at least a 15% market share with Jimpentra alone next year, its second year after launch, separate from Inflectra (marketed as Remsima in the US).
Thomas Nussbickel, Chief Commercial Officer (CCO) of Celltrion USA, said, “With the successful contracts with major PBMs and subsequent listings on formularies of affiliated regional insurers, Jimpentra is establishing itself as a key treatment representing the US inflammatory bowel disease market. Starting next month, we will intensify Jimpentra marketing across all channels, focusing on TV and social media advertising, and expect to secure most of the US coverage within the year, making the 15% market share target achievable.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.