Celltrion announced on the 6th that the results of the global Phase 3 clinical trial analyzing the efficacy and safety of CT-P41, a biosimilar developed from the osteoporosis treatment drug Prolia, have been published in a prestigious international journal. The study results were disclosed through the International Osteoporosis Foundation (IOF) and the Bone Health and Osteoporosis Foundation (BHOF) official journal, International Osteoporosis.
Celltrion conducted a global Phase 3 clinical trial of CT-P41 involving 479 postmenopausal women with osteoporosis across four European countries, comparing the efficacy, pharmacodynamics, pharmacokinetics, and immunogenicity including safety between CT-P41 and the original drug.
The newly published study results cover a 78-week evaluation of the global Phase 3 clinical trial, showing that the primary efficacy and pharmacodynamic endpoints between the CT-P41 and original drug groups met the equivalence criteria. Additionally, efficacy and safety were confirmed in the patient group that switched from the original drug to CT-P41 at week 52.
In detail, the primary endpoint measured was the change in lumbar spine bone mineral density from baseline to week 52 in both the CT-P41 and original drug groups. The results showed that the difference between the two groups met the predefined equivalence criteria. Furthermore, equivalence between the CT-P41 and original drug groups was demonstrated in the area under the curve (AUC) evaluation of the first 6 months' effect on s-CTX, a major bone resorption marker and primary pharmacodynamic endpoint.
Moreover, when comparing three groups?the group that switched from the original drug to CT-P41 at week 52 and the groups that maintained CT-P41 or original drug administration for 78 weeks?the efficacy, pharmacodynamics, pharmacokinetics, and safety evaluation results of the switched group were also found to be similar.
Prolia is approved as an osteoporosis treatment drug and also under the brand name Xgeva for preventing and treating bone metastasis complications in cancer patients. Its global sales last year reached $6.16 billion (approximately 8 trillion KRW).
A Celltrion official stated, “With this announcement, having reconfirmed the efficacy and safety of CT-P41 compared to the original drug, we will do our best to ensure that approvals in major global countries currently underway proceed smoothly. Following our strengths in autoimmune disease treatments and anticancer drugs, we will rapidly expand our therapeutic portfolio in various fields such as bone diseases, eye diseases, and allergic diseases to accelerate growth.”
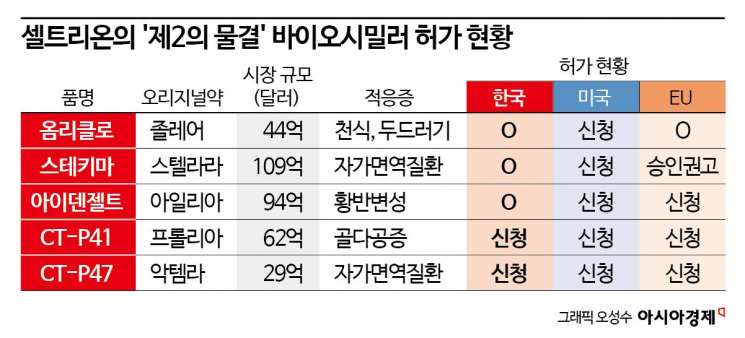
Recently, Celltrion has been accelerating its global market dominance and sales expansion by consecutively obtaining marketing authorizations for follow-up pipelines such as Omriclo (Zolair biosimilar), Stekima (Stelara biosimilar), and Idengelto (Eylea biosimilar).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.