SK Biopharm Secures Radiopharmaceutical Candidate Substance in Hong Kong
Intense Competition for Technology Acquisition Among Novartis, Eli Lilly, and Others
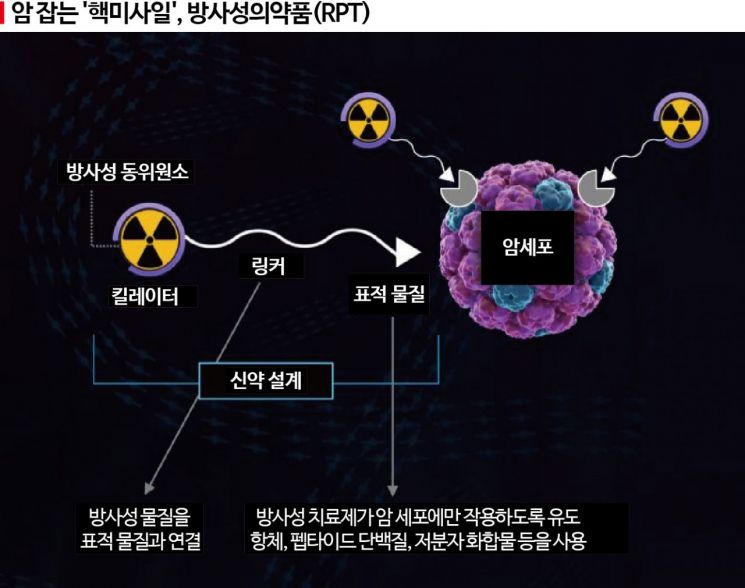
Targeted anticancer therapy technology that selectively kills only cancer cells while leaving normal cells alone is advancing to the level of utilizing radioactive drugs. Pharmaceutical companies both domestically and internationally are investing in this technology.
Antibody-drug conjugates (ADCs), which carry toxic chemical drugs and target specific antigens mainly expressed on cancer cells like “missiles,” currently dominate the global anticancer drug market. Recently, radioactive drug conjugates (RDCs), which load radioactive drugs with much greater cancer cell-killing effects instead of general toxic drugs as “missile explosives,” have begun to attract attention. If ADCs are “conventional guided missiles,” RDCs equipped with radioactive drugs are “guided nuclear missiles.”
SK Biopharm, which is developing RDC anticancer drugs, recently acquired a candidate radioactive drug substance (development name FL-091) to be loaded onto this from Hong Kong-based Fullife Technologies. SK Biopharm will pay Fullife Technologies up to $571.5 million (approximately 792.1 billion KRW) in stages until the finished product of FL-091 is developed, and will pay separate royalties for 10 years after product launch. The profits SK Biopharm can earn are expected to be much greater than this.
Synergies across the entire SK Group are also anticipated. The core radioactive material of FL-091 is actinium-225, and SK Biopharm has secured the right to receive a stable supply of actinium-225 through Terawatt, a small modular reactor company invested in by SK.
RDC is expected to be superior to ADC not only in the firepower to kill cancer cells but also in radar functions to detect cancer cells. While ADC uses antibodies as radar to detect only specific antigens, RDC finds cancer cells through a wider variety of methods, including antibodies and specific proteins expressed only in cancer cells. A representative example is neurotensin receptor 1, a protein expressed in colon and prostate cancer cells. SK Biopharm plans to actively utilize various detection substances besides antibodies in the future development process of RPT.
Lee Dong-hoon, CEO of SK Biopharm, said, "Antibodies can cause side effects because they remain in the body for too long even after killing cancer cells, but radioactive drugs like FL-091 have fewer side effects and are safer."
The global radioactive drug targeted anticancer drug market is expected to grow from $7.9 billion last year at an average annual growth rate of 10.6% to reach $21.8 billion (approximately 30 trillion KRW) by 2033. Novartis is leading the market with Pluvicto, a prostate cancer treatment approved by the U.S. Food and Drug Administration (FDA) in 2022. Last year, it recorded annual sales of $980 million (approximately 1.3583 trillion KRW), a 262% increase compared to the previous year, signaling its rise as a blockbuster treatment.
Other global pharmaceutical companies are also competing to secure related technologies. Eli Lilly signed a contract on the 1st to acquire radiopharmaceutical developer RadiOncotics for $1 billion (approximately 1.386 trillion KRW). Earlier, in October last year, the company acquired Point Biopharma for $1.4 billion (approximately 1.9404 trillion KRW). Bristol Myers Squibb (BMS) recently acquired RaysBio for $4.1 billion (approximately 5.7 trillion KRW), and mergers and acquisitions (M&A) of related companies are actively progressing in the global pharmaceutical market.
Domestically, FutureChem is conducting a Phase 2 clinical trial of FC705, a prostate cancer treatment using radioactive isotopes, aiming for completion in October. It recently received orphan drug designation from the Ministry of Food and Drug Safety. Depending on how the radioactive isotope is used, the drug can also be used for diagnostics, and a Phase 3 clinical trial for prostate cancer diagnosis is also underway. In addition, domestic companies such as Dukem Bio and Selbion are developing anticancer radiopharmaceuticals.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















