Successfully Decomposed Metal-Bonded Water and Toxic Hydrocarbons
Oxidizing Ability Proven... Environmental Protection and Industrial Applications
The UNIST research team has developed a biomimetic catalyst that decomposes harmful hydrocarbon substances.
By oxidizing carbon-hydrogen bonds, this technology is expected to reduce energy consumption and contribute to the prevention of environmental pollution.
The team led by Professor Cho Jaeheung at UNIST (President: Park Jongrae) succeeded in decomposing hydrocarbons from fossil fuels using a catalyst that utilizes water coordinated to metal. This method enables the treatment of hazardous substances under milder conditions compared to conventional approaches. Since it does not require complex processes or high temperatures, it is advantageous for environmental protection.
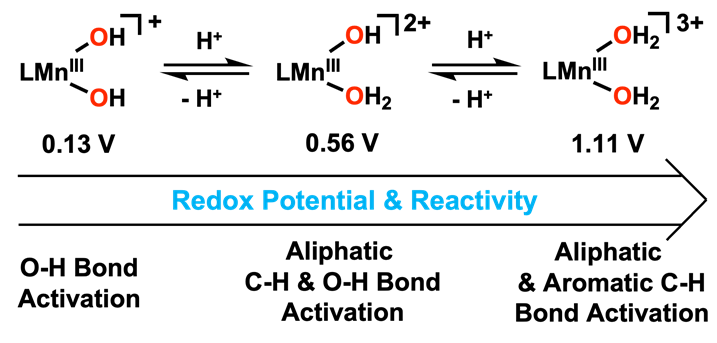
The researchers created a new catalyst by mimicking the ability of natural metalloenzymes to oxidize hydrocarbons. By adding hydrogen ions to a hydroxo ligand composed of oxygen and hydrogen, they synthesized a water molecule coordinated to metal. This method is more efficient and consumes less energy than existing techniques.
The manganese catalyst with added hydrogen ions exhibited improved electron transfer capability. The activation rate of the oxygen-hydrogen bond was also accelerated. This was achieved by replacing the hydroxo ligand with water, thereby increasing the reduction potential of manganese.
Even substances with strong carbon-hydrogen bonds, such as anthracene, were oxidized at low temperatures to eliminate toxicity. The catalyst effectively decomposed aromatic hydrocarbons that are poorly soluble in water and chemically stable.
Professor Cho Jaeheung stated, "This is the first case where a catalyst combining manganese(III) and two water molecules has reacted with aromatic hydrocarbons at low temperatures," adding, "Demonstrating the high oxidation ability to break strong carbon-hydrogen bonds by controlling the reduction potential of manganese will contribute to the development of industrially important metal catalysts."
The research results were published online on June 3 in the Journal of the American Chemical Society (J. Am. Chem. Soc.), a prestigious international journal in the field of chemistry. The study was supported by the National Research Foundation of Korea's Stage-up Carbon Neutral Technology Development Project, DACU Original Technology Development (R&D), and the National New Drug Development Project.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.