Frenzy for Developing Combination Therapies More Effective Than Monotherapy
Tagrisso Approved in US and Korea for Combination with Chemotherapy
Reclaza Completes Combination Trial with J&J's 'Librivant'
Competition in the lung cancer treatment market between AstraZeneca (AZ)'s Tagrisso and Yuhan Corporation's Lekraza is expanding beyond monotherapy to combination therapy. While Tagrisso is taking the lead by obtaining combination therapy approvals first in the United States and Korea, Lekraza is also preparing a counterattack.
 AstraZeneca's non-small cell lung cancer treatment 'Tagrisso (active ingredient Osimertinib)' (left) and Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (Lazertinib)'
AstraZeneca's non-small cell lung cancer treatment 'Tagrisso (active ingredient Osimertinib)' (left) and Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (Lazertinib)' [Photo by each company]
According to Korea AZ on the 18th, the combination therapy of Tagrisso with pemetrexed and platinum-based (cisplatin, carboplatin, etc.) chemotherapy was approved by the Ministry of Food and Drug Safety on the 16th as a first-line treatment for lung cancer. This combination therapy has demonstrated the longest progression-free survival (PFS) period without cancer progression or recurrence after treatment for non-small cell lung cancer with epidermal growth factor receptor (EGFR) mutations to date. The clinical results were first disclosed in September last year and received approval from the U.S. Food and Drug Administration (FDA) in February.
Recently, in the oncology industry, combination therapies that involve multiple treatment methods rather than a single drug monotherapy have gained significant popularity. Especially with the active development of targeted anticancer drugs that attack specific targets related to cancer cells, strategies to complement these and maximize treatment effects are increasing. It is known that about 70% of ongoing global oncology clinical trials are combination therapy trials.
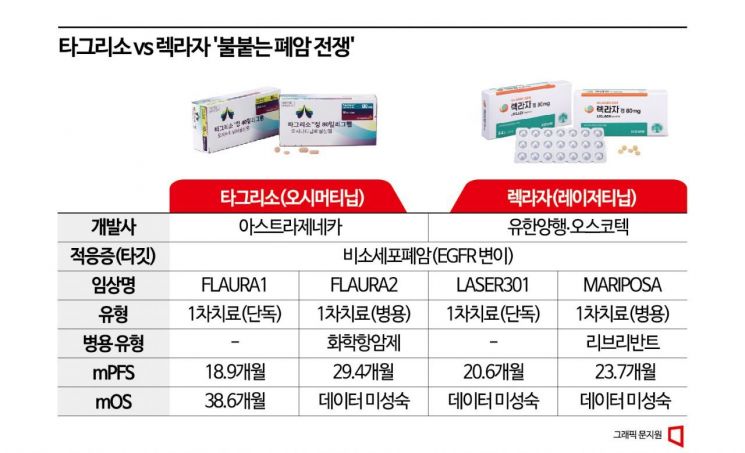
Since both Tagrisso and Lekraza target EGFR-mutated lung cancer, both companies have adopted combination therapies as their next-generation weapons to enhance efficacy. Combination therapies mainly involve using existing standard treatments together or combining targeted therapies that attack related similar targets or immuno-oncology drugs that strengthen the immune system. Among these, Tagrisso chose chemotherapy, the existing standard treatment, while Lekraza selected a similar targeted therapy as its partner.
Both drugs have succeeded in extending PFS compared to existing monotherapies through combination therapies. In the related clinical trial 'FLAURA2,' Tagrisso combination therapy extended the median PFS (mPFS) by about 9 months compared to monotherapy. PFS, along with overall survival (OS), which refers to the period from the start of treatment until the patient's death, is a key evaluation factor for anticancer drug efficacy.
AZ plans to widen the gap with Lekraza, developed by Yuhan Corporation and Oscotec, through this combination therapy, while Johnson & Johnson (J&J), which holds the global rights to Lekraza, is mounting a counterattack. J&J is developing a combination therapy that pairs Lekraza with its targeted antibody therapy, Rybrevant. J&J CEO Joaquin Duato has identified this combination therapy as one of the key pipelines capable of generating annual sales of $5 billion (approximately 6.895 trillion KRW), reflecting high expectations. The results of the 'Mariposa' clinical trial related to this were disclosed last October, and FDA approval is expected to be decided within this year.
However, in the Mariposa trial, although the combination therapy succeeded in securing an mPFS of 23.7 months, longer than Tagrisso monotherapy (16.6 months), it did not reach the 29.4 months achieved by the previously disclosed FLAURA2 combination therapy. Nevertheless, emphasis is placed on the fact that this combination therapy involves a targeted antibody therapy with less patient burden compared to chemotherapy, and since the final OS data has not yet been released, hopes remain high.
Moreover, unlike Lekraza, which has been covered by national health insurance as a first-line treatment in Korea since this year, Rybrevant has not even passed the Cancer Disease Review Committee, the first hurdle for domestic reimbursement. On the 17th, it faced rejection for the third time at the cancer review meeting. Despite already being covered by public insurance in the U.S., Switzerland, Italy, and submitting additional data from actual patient administration domestically, it failed to pass the cancer review. It is analyzed that the fact that it is a treatment for the rare disease EGFR exon 20 mutation, which accounts for only 2% of all lung cancer patients, is hindering its reimbursement approval.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.