UNIST Professor Jeong Sung-kyun's Team Uncovers Thermal Stability Principles of Solid Electrolytes and Cathodes
Proposes New Approach to Improve Thermal Stability... Published in ACS Energy Letters
A breakthrough has been made in developing battery systems that overcome fire and explosion risks.
A domestic research team has found a way to utilize all-solid-state batteries more safely. This study is expected to provide important criteria for the development of safe battery systems.
UNIST (President Yong-Hoon Lee) announced on the 2nd that Professor Sungkyun Jeong’s team from the Department of Energy and Chemical Engineering conducted research on the thermal stability between charged cathodes and halide-based solid electrolytes and revealed their correlation.
The currently most widely used lithium-ion batteries employ organic liquid electrolytes, which pose significant fire and explosion hazards. As an alternative to reduce these risks, all-solid-state batteries (ASSB) using non-flammable inorganic solid electrolytes have attracted attention.
Among inorganic solid electrolytes, sulfide solid electrolytes are being studied as promising materials for next-generation all-solid-state battery development. However, thermal stability issues have persistently arisen due to explosive decomposition products formed between sulfide solid electrolytes and electrodes.
According to UNIST, the research team addressed this issue by utilizing halide-based solid electrolytes. These have superior oxidative stability compared to sulfide solid electrolytes and are mainly used when forming composites with cathodes.
In particular, the team created a composite by mixing LIC (Li3InCl6), the most representative halide solid electrolyte, with a charged NCM622 cathode and conducted an evaluation of thermal stability.
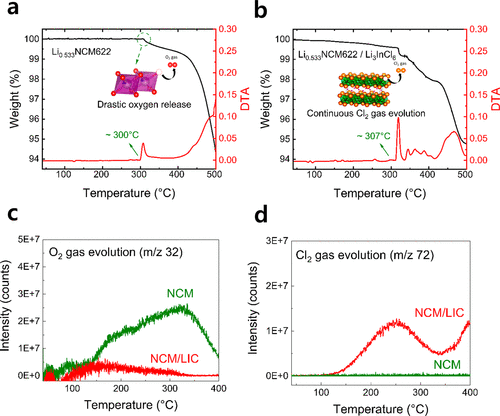
When using sulfide- or oxide-based solid electrolytes, the temperature at which decomposition reactions between the cathode and solid electrolyte begin is lower than when only the solid electrolyte is present. This means they decompose and can explode more easily.
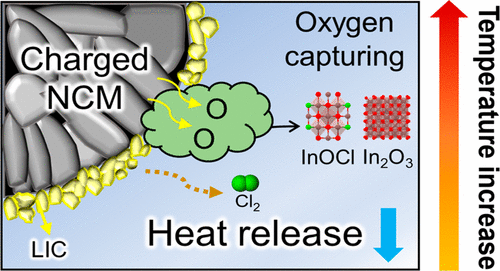
However, the composite of LIC, a halide-based solid electrolyte, and the cathode showed an increased temperature at which decomposition starts, confirming enhanced stability. Additionally, oxygen release, a major cause of explosions, was significantly suppressed.
Through this experiment, the research team also discovered that oxygen generated from the cathode does not turn into gas but disappears through an endothermic reaction with LIC. The same results were confirmed when using other types of halide solid electrolytes such as Li3YCl6 and Li2ZrCl6 or different cathode materials like LCO.
First author researcher Sangpyo Lee said, “This discovery presents a new approach to improving the thermal stability of solid batteries,” adding, “It is expected to provide important criteria for designing safe battery systems in the future.”
Professor Sungkyun Jeong explained, “The research results emphasize that the interaction between solid electrolytes and electrodes plays a crucial role in the thermal stability of all-solid-state batteries,” and added, “It will contribute to the design and development of solid electrolytes for safe battery systems.”
This research was supported by the Ministry of Science and ICT’s Korea Research Foundation Young Researcher Program, the Ministry of Trade, Industry and Energy and the Defense Acquisition Program Administration’s Civil-Military Technology Cooperation Project, and the Korea Institute of Machinery and Materials’ Basic Research Project. It was published online on March 4 in the prestigious international energy journal ‘ACS Energy Letters.’
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.