Pair Eyewear Better Files for Bankruptcy
Major US DTx Companies Face Consecutive Failures
Early Industry Settlement Difficulties in Korea
Digital therapeutics (DTx), known as the "third type of new drug." However, despite the emergence of the "world's first DTx" and leading the market, the industry growth in the U.S. has been sluggish, with a series of bankruptcies occurring. In South Korea, although the first DTx prescription has been made, early growth is facing difficulties as expanding prescriptions proves challenging.
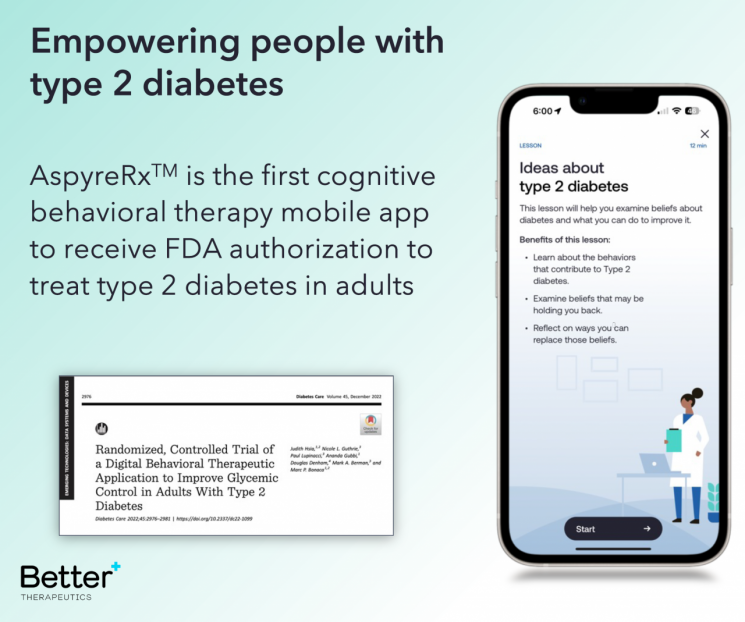
According to the DTx industry on the 26th, Better Therapeutics, a U.S. DTx developer, recently announced plans to delist from Nasdaq. Better plans to lay off employees and sell its assets.
Founded in 2015, Better has struggled financially due to the inability to commercialize products. In 2022 alone, it recorded an operating loss of about $30 million (approximately 40 billion KRW). In March last year, it also underwent a large-scale restructuring, cutting 35% of its workforce. CEO Frank Cabi described it as an "unavoidable decision from a long-term perspective" and expressed hope that the crisis could be overcome through commercialization once the DTx for type 2 diabetes receives market approval. In fact, last July, the type 2 diabetes treatment DTx received FDA approval under the name AspireRx, and the company revealed plans to launch the product in the market within this year. However, it ultimately failed to even cross the threshold of commercialization and faced defeat.
The overall DTx industry is struggling with early growth, contrary to industry expectations. Pear Therapeutics, which developed Reset, the world's first DTx for substance use disorder, filed for bankruptcy in April last year, and game-based DTx developer Akili Interactive also faced difficulties entering the market. DTx emphasizes convenience, allowing treatment anytime and anywhere, rather than superior efficacy compared to traditional treatments like medication or cognitive behavioral therapy. However, as the industry ecosystem has not yet been established, this "cost-effectiveness" has not been accepted in the medical market, leading to ongoing difficulties in maintaining business. Despite Pear holding three FDA-approved DTx products including Reset, it ultimately failed to enter the U.S. federal public insurance system, and actual patient usage rates remained around half, leading to its quiet exit from the market.
"It seems that the companies that took the lead first could not endure," said Kim Chi-won, Managing Director at Kakao Ventures. He added, "The DTx industry is challenging because patients, medical staff, and insurers all need to understand and be satisfied with the product. Given the long time required for the industry to settle, it seems premature to evaluate the entire DTx industry's potential based on cases like Pear and Better."
 In January, Seoul National University Hospital became the first in Korea to prescribe the digital therapeutic device (DTx) Somz.
In January, Seoul National University Hospital became the first in Korea to prescribe the digital therapeutic device (DTx) Somz. [Photo by Seoul National University Hospital]
South Korea is facing similar difficulties in early adoption. In January, AimMed's domestically developed first DTx, Somzz, received product approval and succeeded in treating patients for the first time after about 11 months. However, two months after the start of prescriptions, the cumulative number of prescribed patients is estimated to be around 15. An AimMed official said, "The number of patients was increasing after the start of prescriptions, but the recent full-scale medical crisis has made it difficult to expand prescriptions. Patients who have completed 6 to 8 weeks of treatment continue to emerge, and there have been no cases of treatment discontinuation due to usability issues, so we are monitoring the actual treatment results."
Kang Jae-heon, President of the Korean Society of Digital Therapeutics and professor at Kangbuk Samsung Hospital, said, "South Korea has a national health insurance system, which is better in terms of insurance compared to the U.S., where multiple public and private insurances must be navigated individually. If companies prove the effectiveness and safety of DTx and the National Health Insurance Service takes an active stance, it could contribute not only to industry development but also to public health."
Meanwhile, there is anticipation that DTx treating various diseases beyond mental and neurological disorders such as insomnia will soon emerge in South Korea. NewNaps is developing a DTx to improve visual field defects caused by brain injury, and Share & Service and Life Semantics are preparing DTx to assist respiratory rehabilitation for patients with pulmonary diseases, among other ongoing efforts.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















