Kolon Life Science announced on the 28th that it has filed an appeal with the Supreme Court regarding the cancellation lawsuit of the manufacturing and sales approval for the knee osteoarthritis cell gene therapy product, Invossa-K.
On the 7th, the Administrative Division 10 of the Seoul High Court ruled against Kolon Life Science in the cancellation lawsuit, just as in the first trial, and Kolon Life Science has now filed an appeal against this decision. Through the appeal submitted to the court, Kolon Life Science stated, "We respect the court's ruling, but we will strive to correct the legal misinterpretations and safety assessments made in the appellate court and work to restore the scientific achievements and value of Invossa."
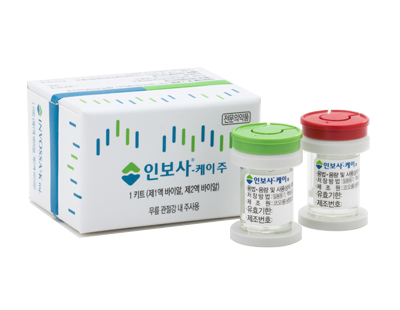
Invossa is an osteoarthritis gene therapy injection composed of two components: Component 1 contains human chondrocytes, and Component 2 contains genetically modified cells introduced with chondrocyte growth factors. It was approved by the Ministry of Food and Drug Safety (MFDS) in 2017 as the first gene therapy product in Korea. However, it was revealed that the genetically modified cells in Component 2 were not chondrocytes but kidney cells that could potentially cause tumors, leading the MFDS to cancel Invossa's approval in 2019.
The company acknowledges that there was a misunderstanding regarding the origin of the cells in Component 2 but claims that all non-clinical and clinical trials were conducted using the same cells prior to the product approval, thus Invossa's safety and efficacy were verified by the MFDS. The company further explained, "The outcome of this administrative lawsuit is completely unrelated to Kolon TissueGene's Phase 3 clinical trial in the United States," and added, "We have decided to proceed with the final trial to correct the misinformation related to Invossa and to restore its scientific value."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















