UNIST Professor Jaeheung Cho's Team Unveils Principles of Catalytic Synthesis and Organic Substrate Oxidation Reactions
Enables Decomposition of Toxic Organic Compounds ... Published in American Chemical Society Journal JACS
A new catalyst with excellent oxidation capability has been developed. Its superior ability to extract electrons from compounds is expected to serve as a cornerstone in various fields, including synthetic chemistry and the development of metal catalysts.
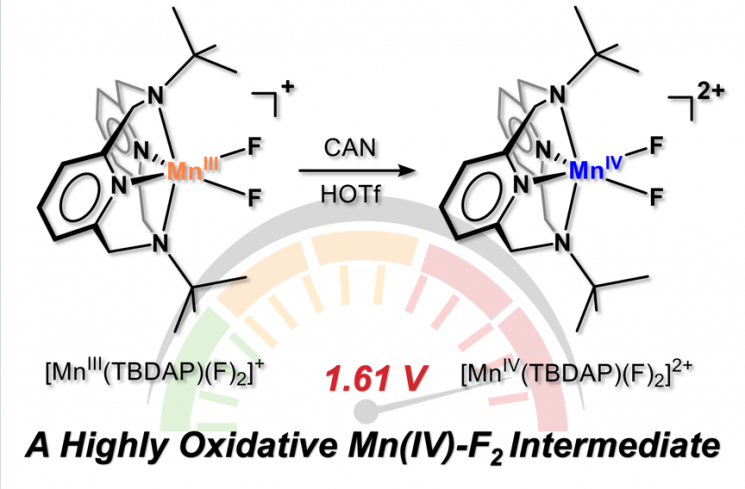
UNIST (President Yong-Hoon Lee) announced on the 20th that Professor Jaeheung Cho's team in the Department of Chemistry successfully synthesized a new manganese-fluorine catalyst.
The research team utilized a macrocyclic pyridino-plane coordination system, where ions or molecules surround atoms in a circular manner and bond. They also confirmed that the developed catalyst can induce oxidation reactions that make toxic substances such as 'toluene derivatives' easily lose electrons.
Professor Jaeheung Cho explained, "The activation of organic compounds with strong carbon-hydrogen bonds was possible due to the characteristics of the manganese-fluorine species, which possess a high reduction potential."
The development of organic catalysts through carbon-hydrogen bond activation is one of the major research areas applicable to pharmaceuticals and industrial processes. In particular, efforts are underway in biomimetic research to imitate the activity of various metal enzymes and create economical metal catalysts.
Recently, metal-halide compounds, where transition metals such as iron and manganese are bonded with halogen atoms like fluorine, have attracted attention as intermediates that oxidize various organic compounds.
The research team newly synthesized a manganese-fluorine catalyst. Among the metal-halide species reported so far, it exhibited the highest reactivity. Increased reactivity allows the decomposition of strongly bonded atoms and their conversion into other compounds. This can be utilized infinitely in various industrial processes.
The team also analyzed the principles of how the developed catalyst induces oxidation reactions. They adjusted the electronic environment of various compounds using the developed catalyst and observed changes in reaction rates. The newly developed catalyst was able to oxidize toluene derivatives with high efficiency, a reaction not observed in existing metal-halide species.
Toluene derivatives are organic substances that can have negative health effects when exposed at high concentrations due to their toxicity. The developed catalyst was confirmed to oxidize toluene derivatives, transforming them into compounds with reduced toxicity.
First authors Donghyun Jeong and Yujeong Lee stated, "This study is the first to report the physicochemical properties of transition metal-fluorine species and newly proposed the principle of carbon-hydrogen bond cleavage based on electron transfer reactions."
 (Third from the left in the back row) Researcher Lee Yuri, Professor Cho Jaeheung in the center. (From the right in the front row) First author Researcher Jung Donghyun, First author Researcher Lee Yujeong.
(Third from the left in the back row) Researcher Lee Yuri, Professor Cho Jaeheung in the center. (From the right in the front row) First author Researcher Jung Donghyun, First author Researcher Lee Yujeong.
Professor Jaeheung Cho said, "This research has great academic significance by proving the high oxidation capability of transition metal-fluorine species," and added, "It is expected to aid in the development of important metal catalysts not only in synthetic chemistry but also in environmental and industrial fields."
This study involved Donghyun Jeong and Yujeong Lee from Professor Jaeheung Cho's research team as co-first authors. The research results were published online on February 4 in the prestigious international chemistry journal, the Journal of the American Chemical Society (JACS).
The research was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Leap Stage Carbon Neutral Technology Development Project, the Academic Successor Support Project, DACU Fundamental Technology Development, and the National New Drug Development Project.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.