Tagrisso Surpasses Reimbursement Threshold 5 Years After Approval
Reclaza Rapidly Closing Gap Just 6 Months Post-Approval
Annual Cost Reduced by 3.4 Million KRW with Reimbursement
Neurofibromatosis Treatment 'Coselugo'
Enters Reimbursement by Skipping Economic Evaluation
Despite being the latest non-small cell lung cancer (NSCLC) treatment, AstraZeneca (AZ)'s Tagrisso (active ingredient osimertinib) had not crossed the threshold for National Health Insurance (NHI) coverage for five years. Finally, its entry into the first-line treatment reimbursement market will take place from the 1st of next month. Alongside this, Yuhan Corporation's Lekraza (lazertinib), developed as a competing new drug, will also see expanded reimbursement, setting the stage for an intense battle for dominance in the NSCLC first-line treatment market starting next year.
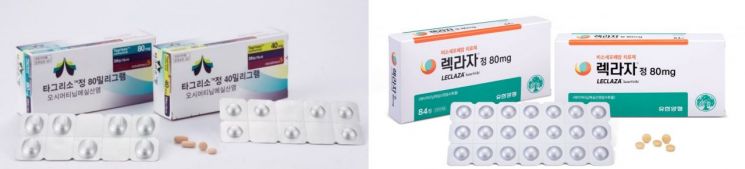 AstraZeneca's non-small cell lung cancer treatment 'Tagrisso (active ingredient Osimertinib)' (left) and Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (Lazertinib)' [Photo by each company]
AstraZeneca's non-small cell lung cancer treatment 'Tagrisso (active ingredient Osimertinib)' (left) and Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (Lazertinib)' [Photo by each company]
On the afternoon of the 20th, the Ministry of Health and Welfare held the 28th Health Insurance Policy Deliberation Committee meeting of 2023 and approved the "Amendment to the Drug Reimbursement List and Reimbursement Ceiling Price Table," which includes these details. The approved content will be applied from January 1st next year after the official notification revision process.
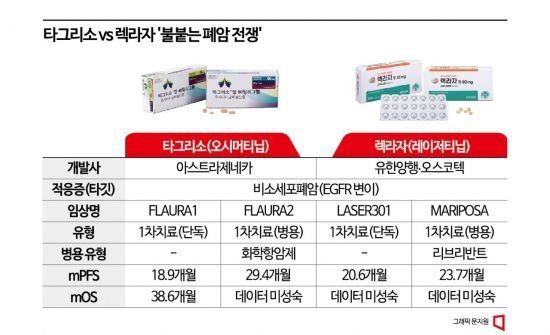
Accordingly, reimbursement for the metastatic NSCLC treatments Lekraza and Tagrisso will be expanded to cover "first-line treatment" for patients with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 (L858R) substitution mutations. Previously, NHI coverage was only applied for second-line treatment, but now it has been extended to first-line treatment, which is the initial therapy. Expanding first-line treatment coverage is crucial because if anticancer drugs are approved only for second- or third-line treatments, patients who are cured or die during first-line treatment would not have the opportunity to use the drugs. This is why developers continuously attempt to expand reimbursement to first-line treatment to reach more patients.
The biggest issue during the expansion of first-line treatment reimbursement was the drug price, which was set similarly at 190,123 KRW per day for Tagrisso (80 mg) and 190,110 KRW per day for Lekraza (80 mg, 3 tablets). These prices represent reductions of 10.0% and 8.1%, respectively, compared to current prices. Patients who have been taking Tagrisso without reimbursement had to bear an annual medication cost of about 68 million KRW per person, but from next year, they will only need to pay about 3.47 million KRW annually (applying a 5% copayment).
However, the actual price reduction could reach up to 50% because the Risk Sharing Agreement (RSA) will be applied. The RSA was introduced to prevent deterioration of health insurance finances amid the launch of high-priced new drugs. It is a system where pharmaceutical companies share some of the uncertainties regarding the drug's effectiveness or insurance financial impact. Additionally, it allows the actual price and the listed price to differ, enabling domestic new drugs to receive higher prices overseas based on the listed price. For example, in the refund-type RSA applied to both drugs, the pharmaceutical company later reimburses a certain percentage of the set price to the National Health Insurance Service.
The expansion of Tagrisso's first-line treatment reimbursement comes five years after the Ministry of Food and Drug Safety (MFDS) approved the indication expansion in 2018. However, it failed to pass the first hurdle for reimbursement expansion, the Cancer Disease Deliberation Committee of the Health Insurance Review and Assessment Service (HIRA), until it finally succeeded on the fifth attempt in March this year. In contrast, the latecomer Lekraza announced the results of the 'LASER 301' clinical trial for first-line treatment expansion in December last year, obtained first-line treatment expansion approval from the MFDS six months later, and passed the cancer deliberation committee two months after that, quickly catching up with Tagrisso. Subsequent procedures such as the Drug Reimbursement Evaluation Committee, price negotiations with the National Health Insurance Service, and the Health Insurance Policy Deliberation Committee proceeded relatively smoothly for Tagrisso as well, seemingly widening the gap, but ultimately, simultaneous reimbursement expansion was decided for both drugs.
With the reimbursement expansion effective from the 1st of next month, Yuhan Corporation's 'Expanded Access Program (EAP)' will also end. Yuhan had been providing Lekraza free of charge to eligible patients through the EAP because reimbursement coverage was not yet applied despite the first-line treatment expansion approval for both drugs. As of the 19th, 864 patients have benefited from this program.
An industry insider said, "Tagrisso has effectively become the standard of care (SoC) for first-line treatment overseas," but added, "With the reimbursement expansion becoming a reality in January next year, both drugs will start on an equal footing, so attention will be focused on the future direction of the domestic SoC."
Meanwhile, AZ's neurofibromatosis type 1 treatment, Koselugo (selumetinib), will also be covered by NHI starting January 1st next year. Neurofibromatosis type 1 is a rare disease characterized by abnormal cell proliferation throughout the body. It is usually diagnosed before the age of 10, and as the body grows, lesions continue to enlarge, often accompanied by complications such as speech disorders, scoliosis, and severe pain. As of 2020, the estimated number of patients in Korea is about 4,000.
Koselugo was approved domestically in May 2021 but had not been reimbursed due to its high cost of 208 million KRW per year. However, the government allowed economic evaluations to be waived for drugs that improve the quality of life in children, and from next year, reimbursement will be applied for pediatric patients aged 3 to 18 with symptomatic, inoperable plexiform neurofibromas associated with neurofibromatosis type 1.
With NHI coverage, the annual medication cost will decrease to 20.8 million KRW when applying a 10% copayment. Additionally, the copayment ceiling system, which refunds the excess amount when annual medical expenses (covered by insurance) exceed a certain threshold to reduce the economic burden on citizens, will enable even high-income groups to reduce their annual medication costs by up to 10.14 million KRW.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















