GC Green Cross 'Aliglo' Approval
Domestic FDA New Drugs Increase to 8
HLB 'Rivoceranib' and Yuhan 'Reclaza' Awaiting Approval
GC Green Cross's immunodeficiency blood product ‘Aliglo’ has received approval from the U.S. Food and Drug Administration (FDA), drawing attention to who will be the next contender. To grow into a global blockbuster, targeting the U.S., the 'world's largest pharmaceutical market,' is the top priority, and various new drugs are stepping up to the challenge.
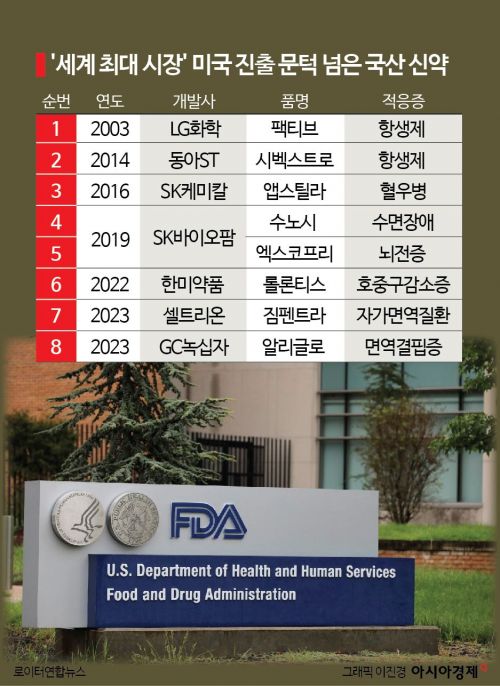
According to industry sources on the 19th, the number of Korean new drugs that have received FDA approval has increased to a total of eight, including Aliglo. Since LG Chem's antibiotic ‘Pactiv’ was first approved by the FDA in 2003, approvals have followed for ▲Dong-A ST’s ‘Cibextro’ (2014) ▲SK Chemicals’ ‘Abstila’ (2016) ▲SK Biopharm’s ‘Sunosi’ and ‘Xcopri’ (2019) ▲Hanmi Pharmaceutical’s ‘Rolontis’ (2022) ▲Celltrion’s ‘Zimpendra’ (2023).
The high regard for FDA-approved new drugs is due to the U.S. being the world’s largest pharmaceutical market, enabling significant revenue generation and providing advantages for entry into other countries. Xcopri achieved sales of 75.7 billion KRW in the third quarter, continuing 14 consecutive quarters of growth since entering the U.S. market. SK Biopharm expects to achieve quarterly profitability in the fourth quarter, buoyed by Xcopri’s strong performance. Zimpendra’s sales, which were 236.9 billion KRW last year, are also expected to grow rapidly starting with its U.S. market entry next year. Celltrion Group Chairman Seo Jung-jin is confident in achieving ‘50 trillion KRW in sales by 2030,’ with Zimpendra’s U.S. market entry as the foundation.
The most likely next FDA-approved new drug is HLB’s anticancer agent containing the active ingredient ‘Lenvatinib.’ In September last year, HLB announced at the European Society for Medical Oncology (ESMO) that the combination therapy of this drug with China’s Hansoh Pharmaceutical’s ‘Camrelizumab’ for liver cancer extended the median overall survival (mOS) to 22.1 months. This far exceeds the existing standard liver cancer treatment’s overall survival (OS) of 12 to 13 months and, as the first liver cancer treatment to surpass an mOS of 20 months, it is expected to establish itself as the ‘best-in-class’ therapy within its category.
 In October last year, the results of the Phase 3 clinical trial of the combination therapy of 'Lenvatinib' and 'Camrelizumab' were announced at the 2022 European Society for Medical Oncology (ESMO) held in Paris, France.
In October last year, the results of the Phase 3 clinical trial of the combination therapy of 'Lenvatinib' and 'Camrelizumab' were announced at the 2022 European Society for Medical Oncology (ESMO) held in Paris, France.
Lenvatinib has submitted its approval application to the FDA, with the final decision expected by May next year. This follows the Prescription Drug User Fee Act (PDUFA) in the U.S., where pharmaceutical companies bear the cost of approval applications in exchange for regulatory agencies processing approvals promptly and responsibly. Once the new drug review application is completed, a final notification deadline of approximately 6 to 10 months is set for the approval result.
Yuhan Corporation’s non-small cell lung cancer treatment ‘Reclaza (Lazertinib)’ is also expected to receive FDA approval as early as next year. Johnson & Johnson (J&J), which acquired the global rights to Reclaza, announced the results of the combination therapy clinical trial ‘Mariposa’ with its targeted antibody treatment ‘Rybrevant (active ingredient Amivantamab)’ at ESMO last October and has expressed plans to submit an approval application within this year. Dr. Cho Byung-chul, head of the Lung Cancer Center at Yonsei Cancer Hospital, who has led the related clinical trials, stated, "Reclaza is not inferior to the competing product Tagrisso," and predicted, "J&J will apply for FDA approval of Reclaza as a monotherapy in the first or second quarter of next year."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["I'd Rather Live as a Glamorous Fake Than as a Poor Real Me"...A Grotesque Success Story Shaking the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















