Mitochondria Present in Cells Throughout the Body
Abnormalities Cause Various Diseases Including Dementia
'Mitophagy' That Removes Damaged Mitochondria Is Important
"First Discovery of Non-Toxic Mitophagy Substance"
Cognitive Recovery Confirmed in Animal Models
26-Year Goal for Human Clinical Trials
"ATL001 is the first mitophagy activator to undergo animal testing. As a dementia treatment, it not only stops cognitive decline but also confirmed cognitive recovery in animal models."
On the 22nd, Yoon Eun-hee, CEO of Alt Medical, who met with Asia Economy, pointed to the 'mitochondria,' an organelle that everyone has trillions of in their body, as the key to new dementia treatments. It is an intracellular organelle known as the 'powerhouse of the cell' because it produces energy through cellular respiration. CEO Yoon explained the importance of mitochondria, saying, "Mitochondria themselves perform various functions," adding, "They contribute to respiration, cell death, immunity, and adenosine triphosphate (ATP) energy conversion."
Conversely, if mitophagy, a type of autophagy that removes damaged mitochondria, does not function properly, cellular functions inevitably deteriorate. More than 50 diseases, including Alzheimer's dementia, Parkinson's disease, and insulin resistance, are known to be related to mitochondrial abnormalities. Therefore, analyses suggesting that dementia treatment through mitophagy is possible have long been proposed. Numerous studies have shown that abnormalities in mitochondrial function lead to the accumulation of substances related to Alzheimer's dementia, such as amyloid-beta (Aβ) and tau (Tau), in the brain, which in turn inhibit mitochondrial function, creating a vicious cycle.
Having worked in the pharmaceutical industry for 30 years at multinational pharmaceutical companies such as Pfizer and Boehringer Ingelheim (BI), CEO Yoon decided to start a company after encountering mitophagy. With aging populations and increasing human lifespans, she thought, "We must ensure that people remain mentally sound up to 100 years old without incurring economic costs due to dementia or other conditions." This naturally brought to mind her university classmate, Professor Yoon Jin-ho of Dong-A University College of Medicine, with whom she had maintained contact. Professor Yoon is globally recognized in the field of mitochondrial research, having received innovation awards from the U.S. National Institutes of Health (NIH). Joining them was Professor Cho Jong-hyun, an expert in organic synthesis-based new drug development, forming what is now Alt Medical.
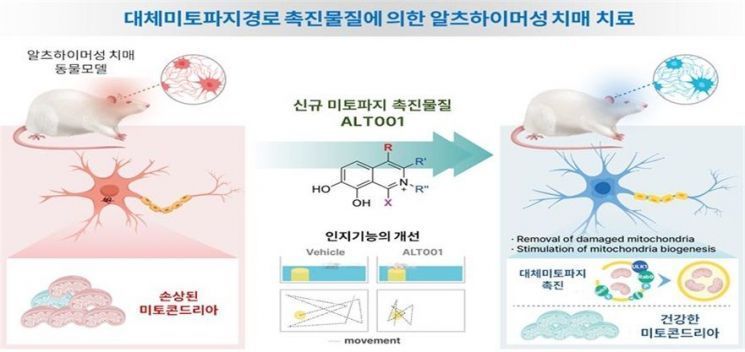 Mechanism illustration of Altmedical's 'ALT001'
Mechanism illustration of Altmedical's 'ALT001' [Photo by Korea Health Industry Development Institute]
However, until now, no treatments utilizing mitophagy have been developed. Although various mitophagy activators have been discovered, strong toxicity often caused cell death even in cell experiments. In contrast, ATL001 developed by Alt Medical has not shown any significant toxicity to date. CEO Yoon explained, "ATL001 selectively targets mitophagy without affecting other autophagy processes and exhibits only mild toxicity," adding, "Alt Medical is the first to confirm efficacy through animal experiments using mitophagy."
Furthermore, while currently developed treatments only slow cognitive decline, CEO Yoon emphasized that ATL001 could be a fundamental treatment that leads to 'cognitive recovery.' She said, "In a current rat model, injecting ATL001 into rats with dementia not only halted cognitive decline but also confirmed cognitive recovery." In animal experiments, cognitive function in rats improved by more than 80%. This confirmed the possibility of a new mitophagy treatment that goes beyond the existing dementia treatment mechanism known as 'ATN,' which refers to Aβ-Tau-neuroinflammation. Regarding the company name, CEO Yoon said, "'Alt Medical' is short for 'Alternative Medical,'" emphasizing, "It reflects our determination to develop a new alternative treatment that goes beyond ATN with mitophagy therapy."
 Yoo Eun-hee, CEO of Altmedical, is explaining the mechanism of ALT001, including mitophagy.
Yoo Eun-hee, CEO of Altmedical, is explaining the mechanism of ALT001, including mitophagy. [Photo by Lee Chun-hee]
ATL001 aims to formally conduct non-clinical trials compliant with Good Laboratory Practice (GLP) standards again next year and enter human clinical trials around 2026. In addition, Alt Medical is developing other mitophagy-based treatments, including 'ALT002,' a new drug candidate for Parkinson's disease, and 'ALT003,' a treatment for chemotherapy-induced peripheral neuropathy (CIPN), a major side effect of anticancer drugs. In the long term, the company plans to continue developing treatments addressing various unmet needs related to mitochondrial dysfunction, including those occurring in outer space.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![From Bar Hostess to Organ Seller to High Society... The Grotesque Con of a "Human Counterfeit" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















