Pneumonia, 3rd Leading Cause of Death in Korea
Free Vaccination Support for Elderly
Pfizer's Prevnar 13 Holds Throne
MSD Challenges with Box Newvans 15-Valent
SK Bioscience Develops 21-Valent Vaccine
With an early winter approaching, concerns about respiratory diseases are also increasing. Among major respiratory diseases, interest in vaccination against pneumonia, which has a significant preventive effect through vaccines, is growing, and new vaccines challenging the existing dominant vaccines are emerging.
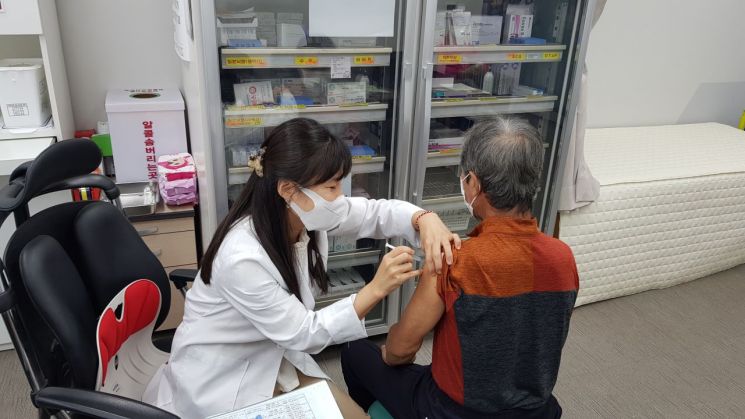 Free pneumococcal vaccinations are being administered at public health centers.
Free pneumococcal vaccinations are being administered at public health centers. [Photo by Damyang-gun]
Pneumonia is a dangerous disease ranking third among causes of death in Korea, following cancer and heart disease. Its mortality rate is even higher than that of brain diseases such as stroke. The most common cause of pneumonia is Streptococcus pneumoniae (pneumococcus). It is known that 27-44% of bacterial pneumonia cases are caused by pneumococcus. It can also lead to complications such as bacteremia and meningitis. Especially with the surge in respiratory virus infections due to the COVID-19 pandemic, the risk of pneumococcal infection has also increased.
Accordingly, the pneumococcal vaccine is recognized for its importance and, along with the influenza vaccine, is an exception to the National Immunization Program (NIP), which is usually targeted at infants and young children, by being applied to adults as well. Since immunity tends to decline with age, people aged 65 and older can receive the vaccine free of charge. In particular, the demand for pneumococcal vaccination is expected to increase significantly as the '58 dog year' cohort, the core of the first baby boom, turns 65 this year. Additionally, the pneumococcal vaccine can be administered year-round regardless of the season. However, Mycoplasma pneumonia, which has recently seen a rapid increase in cases, is caused by Mycoplasma bacteria rather than pneumococcus, making it difficult to prevent with the pneumococcal vaccine, and a vaccine targeting Mycoplasma bacteria has not yet been developed.
The undisputed leader in the domestic pneumococcal vaccine market is Pfizer's 'Prevnar 13.' Last year, it recorded sales of 40.9 billion KRW, boasting an overwhelming market share of 70-80%. MSD (Merck & Co., USA) has challenged this with its 15-valent vaccine 'Vaxneuvance.' Following FDA approval in the United States in 2021, it was approved domestically last month. It already achieved sales of 138 million USD (approximately 180 billion KRW) overseas last year. In addition to the 13 serotypes covered by Prevnar (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F), Vaxneuvance has expanded its coverage by adding the ‘22F’ and ‘33F’ serotypes, which have recently been identified worldwide as major causes of pneumococcal diseases.
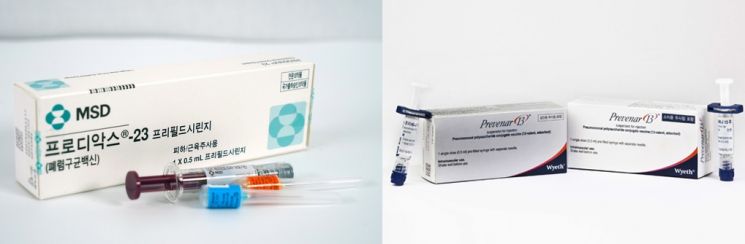 MSD's pneumococcal vaccine 'Prodiac23' (left) and Pfizer's pneumococcal vaccine 'Prevnar13'
MSD's pneumococcal vaccine 'Prodiac23' (left) and Pfizer's pneumococcal vaccine 'Prevnar13' [Photo by MSD Korea, Pfizer Korea]
Although 23-valent vaccines have been used domestically, Vaxneuvance boasts the 'highest valency approved domestically' because it is a different type of vaccine. The existing 23-valent vaccines are polysaccharide vaccines (PPSV), whereas Vaxneuvance and Prevnar are protein-conjugate vaccines (PCV). PPSV is made using polysaccharides from the capsule outer wall of pneumococcus, while PCV conjugates polysaccharides with a protein carrier to induce T-cell dependent immune responses. PPSV covers more serotypes, allowing broader protection against various strains, whereas PCV covers fewer serotypes but is known to have higher preventive efficacy. Currently, PPSV 23-valent vaccines are used for elderly vaccination through the NIP, and PCVs (10-valent and 13-valent) are used for infant and pediatric vaccination. Experts explain that sequential vaccination with PCV13 followed by PPSV23 is the most effective method for prevention.
PCV developers are rapidly increasing the number of serotypes covered. The current highest valency for PCV is 20-valent. Pfizer's 'Prevnar 20' received FDA approval in the United States in 2021. It successfully reclaimed the position of highest valency, which had been temporarily taken by Vaxneuvance, and quickly dominated the market after its launch, boasting an overwhelming market share of over 90% in the U.S. It has also applied for approval from the Korean Ministry of Food and Drug Safety.
 At the Vaccines Investor Event hosted by Sanofi in London, UK, last June, Jaeyong Ahn, President of SK Bioscience (left), and Thomas Triomphe, Senior Vice President of Sanofi's Vaccine Division, posed for a commemorative photo. At the event, the two companies disclosed the Phase 2 clinical trial results of the 21-valent protein-conjugate vaccine "SkyPack," which they are jointly developing.
At the Vaccines Investor Event hosted by Sanofi in London, UK, last June, Jaeyong Ahn, President of SK Bioscience (left), and Thomas Triomphe, Senior Vice President of Sanofi's Vaccine Division, posed for a commemorative photo. At the event, the two companies disclosed the Phase 2 clinical trial results of the 21-valent protein-conjugate vaccine "SkyPack," which they are jointly developing. [Photo by SK Bioscience]
Domestically, SK Bioscience is focusing on developing pneumococcal vaccines. It previously succeeded in developing Korea's first pneumococcal PCV13 vaccine, 'SkyNemo.' However, SkyNemo is not being sold due to patent infringement issues with Pfizer. Therefore, SK Bioscience is accelerating the development of PCV21 'SkyPack (GBP410),' which covers the highest number of serotypes among PCVs and aims to be the 'best-in-class' within its category. The vaccine has been licensed out to Sanofi and demonstrated excellent immunogenicity and safety in Phase 2 clinical trials. It plans to enter Phase 3 clinical trials in the first half of next year and aims to begin the approval process by 2027. MSD is also developing a PCV21 vaccine called 'V116.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

















