Reclaza Combination Therapy 'Mariposa' Clinical Trial
Market Doubts Grow Over Ambiguous Key Indicators
Professor Jo Byung-chul Leading Reclaza Clinical Trial
"Differences Due to Experimental Methods... Actual Effectiveness Is the Same"
"Need to See If Adverse Reactions Can Be Managed and Overcome"
"The improvement in median progression-free survival (mPFS) of the combination therapy of Rybrevant and Lekraza should be considered as 9 months."
"Johnson & Johnson (J&J) will also submit a New Drug Application (NDA) for Lekraza monotherapy in the first or second quarter of next year."
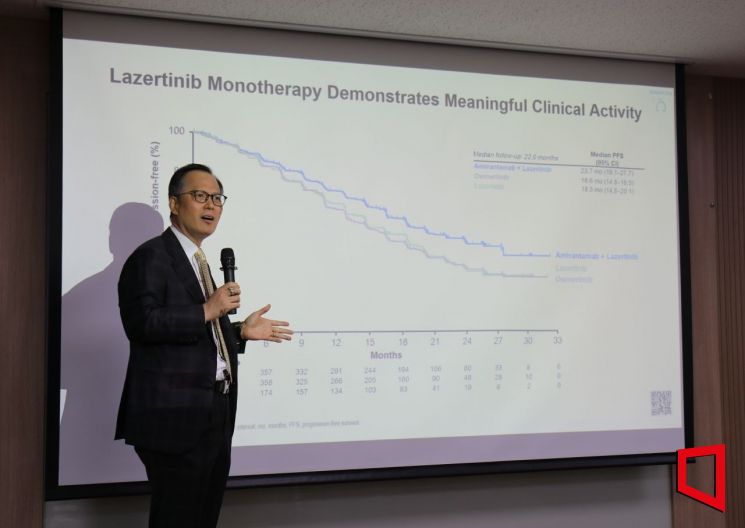 Jo Byung-chul, Director of the Lung Cancer Center at Yonsei Cancer Hospital, is explaining the 'MARIPOSA' clinical trial at a briefing held on the 27th in Seocho-gu, Seoul.
Jo Byung-chul, Director of the Lung Cancer Center at Yonsei Cancer Hospital, is explaining the 'MARIPOSA' clinical trial at a briefing held on the 27th in Seocho-gu, Seoul. [Photo by Lee Chun-hee]
On the 27th, at a briefing held in Seocho-gu, Seoul, Cho Byung-chul, head of the Lung Cancer Center at Yonsei Cancer Hospital, spoke about the clinical trial results of the combination therapy of Yuhan Corporation's 'Lekraza (active ingredient: Lazertinib)' and J&J Innovative Medicine (formerly Janssen)'s targeted antibody therapy 'Rybrevant (Amivantamab)' in the 'MARIPOSA' clinical trial, emphasizing once again that Lekraza has the potential to become a global blockbuster drug.
Professor Cho has been deeply involved in the development of Lekraza since Yuhan Corporation licensed it from Oscotec in 2015, leading related clinical trials. He also served as the principal investigator for the MARIPOSA trial.
More Detailed Patient Observation in 'MARIPOSA': "Uneven Playing Field... Direct Comparison Difficult"
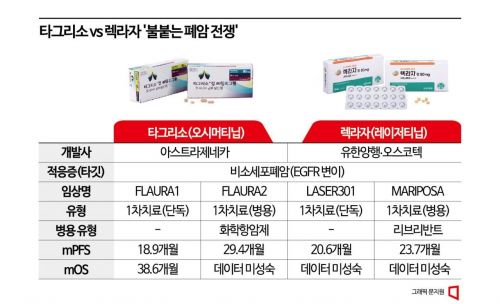
Regarding the MARIPOSA clinical trial results announced by Professor Cho on the 23rd (local time) at the '2023 European Society for Medical Oncology (ESMO 2023)' in Madrid, Spain, criticism has arisen that the results did not surpass AstraZeneca (AZ)'s 'Tagrisso (Osimertinib)', the current standard of care (SoC) for non-small cell lung cancer. The MARIPOSA trial achieved its primary efficacy endpoint with an mPFS of 23.7 months, extending by 7 months compared to the Tagrisso monotherapy group’s 16.6 months.
However, compared to the 'FLAURA 2' trial, which combined Tagrisso with chemotherapy and reported mPFS results of 25.5 months or 29.4 months (according to the independent central review committee), the MARIPOSA trial’s mPFS of 23.7 months fell short of the market’s expectation of 25 months. Additionally, the PFS extension of the MARIPOSA trial was considered shorter than the 8.8-month PFS extension achieved in FLAURA 2.
Nonetheless, Professor Cho emphasized that the results should not be directly compared one-to-one due to differences in study design, calling it an "uneven playing field." He explained, "In the MARIPOSA trial, all patients underwent brain magnetic resonance imaging (MRI) and abdominal and chest computed tomography (CT) scans every 8 weeks," adding, "This was to observe the effects of the combination therapy more precisely and to monitor the time to brain metastasis." Because the examination intervals were more frequent, progression-free survival (PFS) naturally appeared shorter as tumors were detected earlier during scans.
This is also why Professor Cho focuses on 'extracranial PFS.' He mentioned, "If the examinations were conducted like in other studies, the mPFS of the Lekraza-Rybrevant combination therapy would increase to 27.5 months, which is about 9 months longer than Tagrisso’s 18.5 months." He added, "Just like the 8.8-month difference in PFS extension confirmed in FLAURA 2 between Tagrisso and chemotherapy combination therapy, the PFS extension effect is similar whether it is Rybrevant or chemotherapy." Furthermore, he highlighted the importance of the therapy by noting that consistent data were observed across detailed subgroups such as ethnicity, weight, smoking history, brain metastasis, and EGFR mutation status, stating, "There is no criterion to decide which patients should use this therapy more or less."
He repeatedly emphasized the overall survival (OS) data to be disclosed later. Since the median OS (mOS) is calculated only after more than half of the trial participants have died, the OS data for MARIPOSA have not yet been released. Professor Cho said, "The hazard ratio (HR) was 80% in the interim analysis, and if statistical significance (p-value) is achieved, OS benefits will be confirmed," adding, "At JP Morgan, it was also seen as possible to secure a 'significant market share'." On the other hand, regarding chemotherapy combination therapy, he noted, "Many doctors will abandon it unless OS benefits are recognized," and added, "The FLAURA 2 trial did not show a trend indicating such benefits."
 Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (active ingredient: Lazertinib)'
Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (active ingredient: Lazertinib)' [Photo by Yuhan Corporation]
Regarding safety concerns, Professor Cho conveyed that they are sufficiently manageable. In the MARIPOSA trial, grade 3 or higher adverse events occurred in 75% of the combination therapy group, higher than the 43% in the Tagrisso group. He explained that the 75% figure includes reactions unrelated to the drug, such as traffic accidents, and emphasized that the actual rate of patients who discontinued all medications due to drug-related adverse events was about 10% in the combination group.
He also noted that even among grade 3 or higher adverse events, skin conditions such as paronychia, skin rash, and acneiform rash were predominant, stressing that "these are not life-threatening." In contrast, the Tagrisso-chemotherapy combination therapy showed about 20% of grade 3 or higher adverse events related to life-threatening conditions such as anemia, neutropenia, and thrombocytopenia.
Professor Cho stated, "There is hardly any therapy where toxicity does not increase when combination therapy is used," and added, "(Side effects like skin rash) can be actively managed, and patients choose to live longer rather than give up because management is bothersome." He emphasized the importance of carefully evaluating whether adverse events are truly frequent and whether they are manageable side effects.
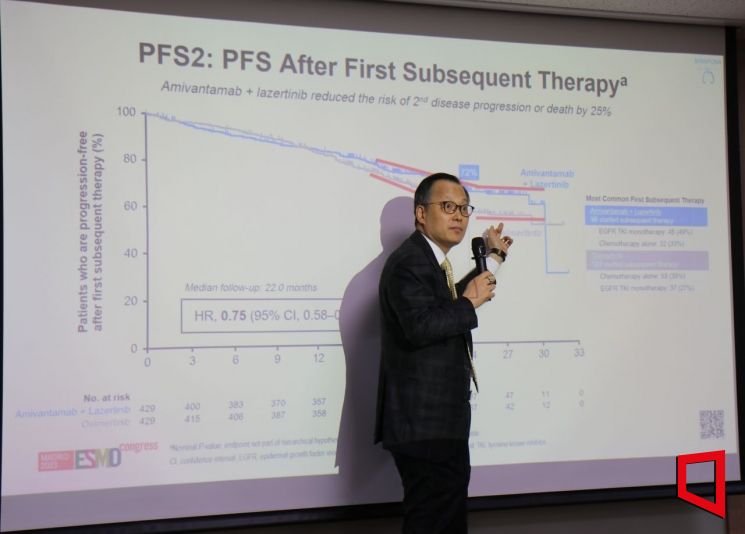 Jo Byung-chul, head of the Lung Cancer Center at Yonsei Cancer Hospital, is explaining the 'MARIPOSA' clinical trial at a briefing held on the 27th in Seocho-gu, Seoul.
Jo Byung-chul, head of the Lung Cancer Center at Yonsei Cancer Hospital, is explaining the 'MARIPOSA' clinical trial at a briefing held on the 27th in Seocho-gu, Seoul. [Photo by Lee Chun-hee]
Professor Cho also expressed optimism about the results of the Lekraza monotherapy group included in this trial. The trial included not only the Lekraza-Rybrevant combination therapy group (429 patients) and the Tagrisso monotherapy group (429 patients) but also a Lekraza monotherapy group (216 patients) for comparative analysis. The mPFS of the Lekraza monotherapy group was 18.5 months, about two months longer than the Tagrisso monotherapy group’s 16.6 months.
Previously, Lekraza showed about a two-month PFS extension effect compared to Tagrisso in the 'LASER 301' trial for monotherapy approval, with an mPFS of 20.6 months versus Tagrisso’s 18.9 months (FLAURA 1). Professor Cho explained, "Lekraza is clearly not inferior to Tagrisso," adding, "This was also achieved because MRI and CT scans were conducted frequently, so the effect was better than 20.6 months."
He said, "J&J will submit an NDA for Lekraza monotherapy in the first or second quarter of next year," adding, "They will apply by combining the data from LASER 301 and the Lekraza monotherapy group in the MARIPOSA trial." Currently, Lekraza monotherapy is approved as a first-line treatment in Korea and is attempting to enter the National Health Insurance reimbursement system. Yuhan Corporation is conducting an 'Expanded Access Program (EAP)' to provide Lekraza free of charge to patients until reimbursement is secured. As of this date, more than 500 patients have participated in the EAP, and Yuhan expects that the number of supported patients will exceed 1,000 by the end of the year if this trend continues.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















