Evaluation as a Prequel to Next Year's JPM by the '3 Major Cancer Societies'
Yuhan's 'Reclaza' and 'Mariposa' Clinical Trials to Be Disclosed
Significant Improvement Expected Compared to Tagrisso
Hanmi, HLB, GI, SillaJen, Lunit, and Others Also Participating
The annual meeting of the European Society for Medical Oncology (ESMO), considered one of the world's top three cancer societies, will kick off this week. With various domestic and international companies planning to disclose research data, attention is focused on whether Korean companies can present remarkable achievements at this last major cancer conference of the year.
 2023 European Society for Medical Oncology (ESMO) Annual Congress
2023 European Society for Medical Oncology (ESMO) Annual Congress [Photo by European Society for Medical Oncology]
This year's 'ESMO 2023' will be held from the 20th to the 24th in Madrid, Spain. It is the largest cancer conference in Europe and is regarded as one of the world's top three cancer societies alongside the American Society of Clinical Oncology (ASCO) and the American Association for Cancer Research (AACR). As the last major event held annually, it is also considered a precursor to the following year's JP Morgan Healthcare Conference (JPMHC), the world's largest venue for technology transfer and investment attraction.
This ESMO will feature presentations of research results from companies such as Yuhan Corporation, Hanmi Pharmaceutical, HLB, GI Innovation, SillaJen, and Lunit. Notably, last year HLB announced an innovative achievement where 'Lenvatinib' combined with Antengene's 'Camrelizumab' in a Phase 3 clinical trial showed a median overall survival (mOS) of 22.1 months, surpassing 20 months for the first time in first-line treatment of liver cancer, drawing attention to whether similar major announcements will be made this year.
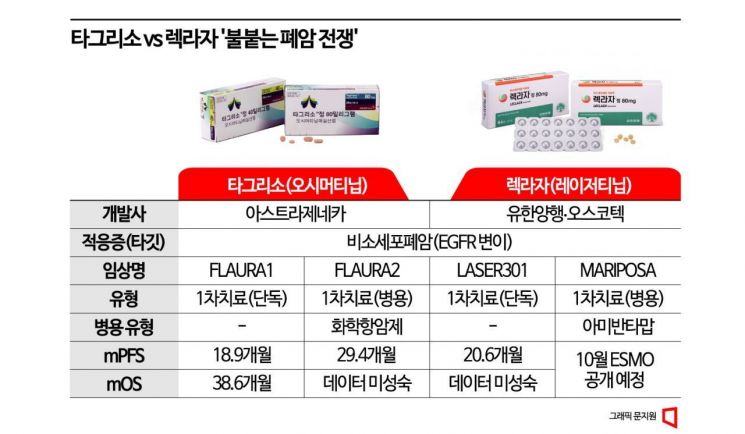
Will Yuhan Corporation's 'Leclaza' catch up to Tagrisso through the 'Mariposa' clinical trial?
The company most highly regarded for this potential is undoubtedly Yuhan Corporation. Johnson & Johnson (J&J) Innovative Medicine (formerly Janssen), which licensed Yuhan's non-small cell lung cancer treatment new drug 'Leclaza (Lazertinib)', is scheduled to present results from the combination clinical trial 'Mariposa' with its own targeted antibody therapy 'Libtrivant (Amivantamab)'.
Last month, J&J announced that the progression-free survival (PFS) of the Leclaza and Libtrivant experimental group was "statistically significant and clinically meaningful" compared to the existing first-line standard treatment 'Tagrisso (Osimertinib)' monotherapy group, raising expectations for the final results disclosure. PFS refers to the period during which cancer does not progress after treatment and, along with overall survival (OS), which measures the time from treatment start to patient death, is considered a key indicator for evaluating anticancer drug efficacy.
With the news of clinical success, there is growing expectation that Leclaza could rapidly catch up to Tagrisso, which has been internationally recognized as the preferred first-line treatment, raising hopes that it will become Korea's first global blockbuster drug. In the pharmaceutical and biotech industry, a drug generating annual sales exceeding $1 billion (approximately 1.349 trillion KRW) is considered a blockbuster. J&J CEO Joaquin Duato has identified this therapy as one of the key pipelines capable of generating over $5 billion (approximately 6.745 trillion KRW) in annual sales. Eugene Investment & Securities analyst Kwon Hae-soon also analyzed that considering royalties, profits generated by Leclaza could approach 1 trillion KRW by 2030. J&J is known to plan to quickly initiate FDA approval procedures following this ESMO presentation, aiming to obtain FDA approval within next year.
Hanmi Pharmaceutical will disclose results from a domestic Phase 1b clinical trial of the combination therapy of the pan-RAF inhibitor targeted anticancer drug 'Velbarafenib' and Roche's MEK inhibitor 'Cotellic (Cobimetinib)'. Velbarafenib was licensed out in 2016 to Genentech, a subsidiary of Roche, and has since been involved in various combination clinical trials with Roche and Genentech's anticancer drug pipelines. At last year's ASCO, the combination therapy of Velbarafenib and Cotellic showed a partial response (PR) rate of 26.3% in NRAS-mutant melanoma patients, with a median progression-free survival (mPFS) of 7.3 months.
HLB, which rose as a star at last year's ESMO with the combination clinical trial announcement of Lenvatinib, will disclose clinical data on Lenvatinib for gastric cancer. This will also reveal the first interim analysis results of a triple combination clinical trial combining Antengene's Camrelizumab and chemotherapy. GI Innovation will present results from Phase 1 and 2 monotherapy clinical trials of 'GI-101', and SillaJen will disclose Phase 2 combination clinical trial results of the oncolytic virus 'Pexa-Vec' with Regeneron's immune checkpoint inhibitor 'Libtayo' in kidney cancer patients.
Lunit will present nine abstracts, mainly revealing results that improved predictive efficacy in various cancer diagnoses and treatments using its artificial intelligence (AI) biomarker 'Lunit Scope'. In a study on progressive cholangiocarcinoma patients' response to anti-PD-1 therapy, using Lunit Scope to classify tumor-infiltrating lymphocytes (TIL) distribution into immune-active, immune-excluded, and immune-deficient groups, the immune-active group showed better outcomes with OS of 12.5 months, PFS of 5.0 months, and objective response rate (ORR) of 27.5%, compared to the immune-deficient group's 5.1 months, 2.0 months, and 7.7%, respectively.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.