HIRA Ultra-High-Cost Drug Patient Response Evaluation Data
Most of the Zolgensma Injections, Costing Up to 2 Billion KRW, Show Improvement Effects
Kimira Injection and Zolgensma Injection Covered by National Health Insurance Since April and July Last Year, Respectively
If Ultra-High-Cost Drugs Are Ineffective for Patients, Partial Reimbursement of Coverage Costs Required
"Reimbursement Rate Should Be Increased If Treatment Is Ineffective"
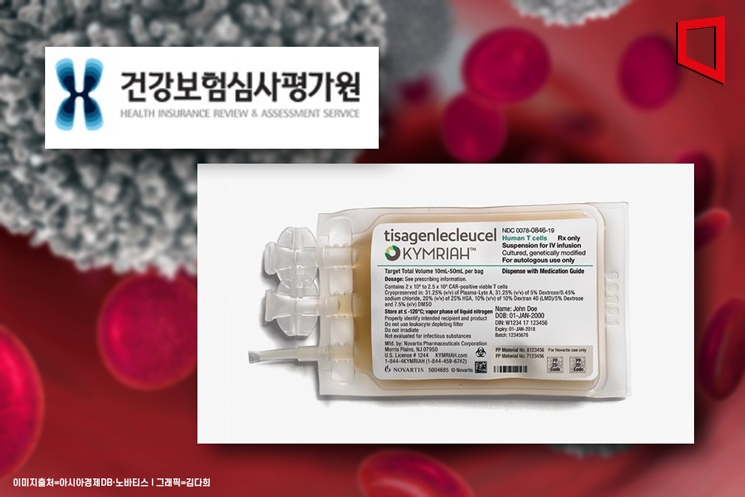
Among patients treated with the leukemia and lymphoma drug Kymriah (active ingredient tisagenlecleucel), which costs up to 400 million KRW, 7 to 8 out of 10 showed little effect. In contrast, most patients treated with the ultra-expensive spinal muscular atrophy (SMA) drug Zolgensma (active ingredient onasemnogene abeparvovec), which costs up to 2 billion KRW, experienced therapeutic benefits.
On the 6th, Kim Young-joo, a member of the National Assembly’s Health and Welfare Committee (Democratic Party), analyzed data titled “Status of Ultra-High-Cost Drug Administration and Patient Response Evaluation for Kymriah and Zolgensma” received from the Health Insurance Review and Assessment Service. As of August, among 130 lymphoma patients who had been treated with Kymriah for more than six months, 99 were classified as eligible for reimbursement. This means that over 76% of Kymriah-treated patients showed no improvement.
However, there are also opinions that it is premature to conclude treatment outcomes based solely on current data, as many severe patients were enrolled during the initial phase of Kymriah’s insurance coverage. A representative from Novartis Korea explained, “When Kymriah was covered by insurance last year, many severe lymphoma patients with an expected survival of only six months began treatment. This may have made the effectiveness appear lower. In countries like Japan, where approval was granted earlier than in Korea, real-world clinical results have sometimes shown higher effectiveness than clinical trials.”
Developed by global pharmaceutical company Novartis, Kymriah is a treatment for acute lymphoblastic leukemia and diffuse large B-cell lymphoma. It is primarily used as a “one-shot therapy” for patients under 25 years old, but the cost per single administration reaches 360 million KRW. To provide treatment opportunities to patients, the Ministry of Health and Welfare decided last April to apply health insurance coverage to Kymriah. Patients only need to pay the health insurance out-of-pocket maximum of 5.98 million KRW to receive the treatment.
On the other hand, Zolgensma, a spinal muscular atrophy (SMA) treatment costing 1.98 billion KRW per administration, was analyzed to have a high treatment effect. Among nine patients who submitted patient response evaluations, only one was eligible for reimbursement, meaning over 88% experienced therapeutic benefits. Developed by Novartis, Zolgensma was covered by health insurance starting last July.
For ultra-high-cost drugs for severe diseases, a “patient-level performance-based risk-sharing scheme” is applied, where pharmaceutical companies refund a certain percentage of the cost to the National Health Insurance Service if the drug is ineffective for individual patients. This is intended to prevent excessive health insurance claims despite reduced drug efficacy.
As of June, the total insurance claims for Kymriah and Zolgensma amounted to 76.4 billion KRW, with Kymriah accounting for 52.6 billion KRW and Zolgensma 23.8 billion KRW. While the refund rate for Kymriah is below 50% even if the drug is ineffective, Zolgensma’s refund rate is known to be above 50%.
Assembly member Kim Young-joo stated, “To prevent blind spots, insurance coverage should be expanded for patients who need ultra-high-cost treatments like Kymriah and Zolgensma.” However, she advised, “Since hundreds of billions of won are spent on drugs with low treatment success rates, to ensure sustainable insurance coverage for ultra-high-cost new drugs, it is necessary to strengthen the performance-based risk-sharing scheme and increase the refund rate from pharmaceutical companies when there is no therapeutic effect.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.