Interview with Lee Seungha, Head of Production Center at Daewoong Pharmaceutical
Osong Plant Built as a 'Smart Factory'
Complete 'Data Integration' Preventing Data Manipulation
Aiming for Full Automation by Achieving 'Smart Level 5'
"As a smart factory, we have successfully passed domestic and international inspections in terms of quality, and productivity indicators have improved by 40%. In the long term, we aim to achieve Level 5 of the smart factory and become a 'leading factory' that creates a standard model for future pharmaceutical production plants."
 Seungha Lee, Head of Production Division at Daewoong Pharmaceutical (Osong Plant Manager) [Photo by Chunhee Lee]
Seungha Lee, Head of Production Division at Daewoong Pharmaceutical (Osong Plant Manager) [Photo by Chunhee Lee]
Seungha Lee, Head of Production Headquarters and Plant Manager of Daewoong Pharmaceutical Osong Plant, emphasized that the Osong Plant is an error-free 'smart factory' through automation and presented this goal. Regarding the Daewoong Pharmaceutical Osong Plant, which was completed in 2017 with a total investment of 210 billion KRW aimed at becoming a smart factory, he said, "The main directions of a smart factory are automation, unmanned operation, and digitalization." He added, "We secure quality through various IT solutions that fundamentally prevent human errors in each manufacturing process and implement data management capabilities that exceed global standards. At the same time, we aim to become a factory that creates a super-gap in competitiveness by fostering highly trained experts, securing high-level quality competitiveness and cost manufacturing competitiveness through a production system that prevents human errors, a digital data management system that is tamper-proof, and real-time environmental and process monitoring systems."
In particular, regarding quality, the plant manager repeatedly emphasized that the process status can be monitored and controlled in real time, and data tampering is impossible. He explained, "The Osong Plant operates on a paperless factory concept where manufacturing test records and all logs are automatically recorded and managed," and "All IT systems necessary for plant operation are established, enabling real-time monitoring and control of the process status from raw material receipt to finished product shipment."
The achievements of the smart factory were also proven through a recent manufacturing and quality-related inspection by Brazil's National Health Surveillance Agency (ANVISA). The plant manager said, "During the inspection conducted from the 4th to the 7th, we received feedback of 'Zero Observation' from the local partner and inspectors," and added, "We expect to receive the certification by next month." He also hinted, "In terms of productivity, productivity indicators improved by 40%, and the cost ratio was reduced by more than 30%."
He emphasized that the smart factory greatly benefited the ANVISA inspection process. Highlighting that "data integration" is the most important aspect of Good Manufacturing Practice (GMP) certification, he said, "We have a system where data modification or alteration is impossible. Without such a system, we would have to explain with many documents, but once the inspectors understood the system's feature of being tamper-proof, the difficulty of the inspection eased."
The plant manager also cited Daewoong Pharmaceutical's internalization of IT development capabilities as a major advantage in building such a smart factory. Daewoong Pharmaceutical currently owns a subsidiary, ids&Trust, which provides digital healthcare solutions. All systems used at the Osong Plant, including the Laboratory Information Management System (LIMS), were developed by ids&Trust. He emphasized, "I believe pharmaceutical companies understand the pharmaceutical industry best," and added, "We created specialized solutions with an accurate understanding of the pharmaceutical industry, and other companies actually purchase and operate these solutions, demonstrating considerable competitiveness."
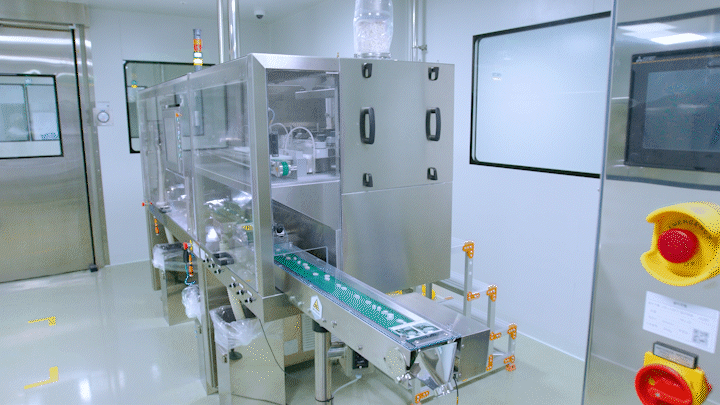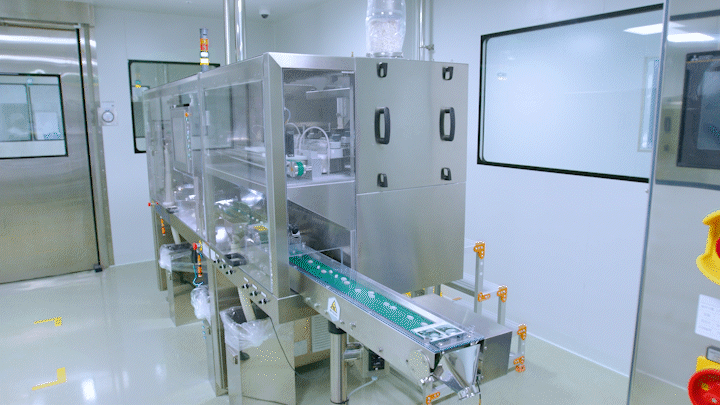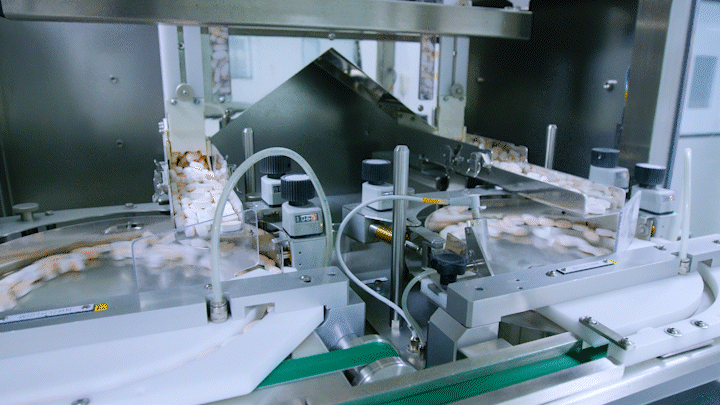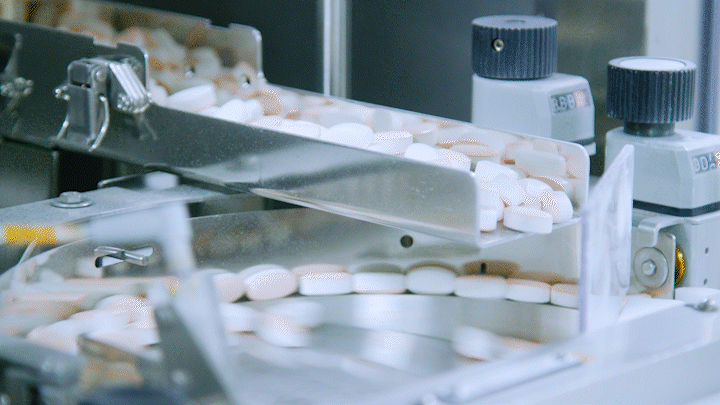 Medicines are being automatically produced at Daewoong Pharmaceutical Osong Plant.
Medicines are being automatically produced at Daewoong Pharmaceutical Osong Plant. [Photo by Daewoong Pharmaceutical]
The next goal for the Osong Plant is Level 5, the highest stage of a smart factory. After achieving Level 3 in 2019, it received Level 4 certification last year. Level 4 allows for preemptive response and decision-making optimization through simulation, and if there are no errors or process changes, most processes require no human intervention. Reaching the final Level 5 means autonomous monitoring, control, and optimization, with AI capable of handling process changes and error responses in real time, representing complete unmanned and fully automated operations.
The plant manager said, "In fact, I believe that AI making decisions like at Level 5 still poses risks in the pharmaceutical industry," but added, "Under the goal of becoming a leading factory and a global standard factory, we have a grand vision to achieve Level 5." To this end, he also set a goal to achieve complete unmanned operation of the tablet compression process within three years. Tablet compression refers to the process of converting granulated and mixed materials into the pill form we consume. He said, "We believe that tablet compression and sorting can be operated through AI by developing sufficient algorithms," and added, "We plan to achieve tablet compression within three years, sorting within five years, and complete automation of all processes within 10 to 20 years, building them step by step."
Furthermore, since the plant already possesses considerable competitiveness in quality, it also expressed the intention to challenge GMP certifications from other regulatory agencies in the future. The plant manager said, "Obtaining ANVISA's GMP, which we are almost expecting, is a significant achievement," and added, "In line with the overseas market entry strategies for key products such as Pexuclu and Enblo, we are fully capable of challenging GMP certifications in the US, Europe, and other regions."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

















