KAIST-KIST Joint Research Team
High Durability with Catalyst Usage Reduced to Less Than One-Tenth
A technology that utilizes semiconductor technology capable of implementing a three-dimensional network structure to maintain high hydrogen production efficiency over a long period has been developed.
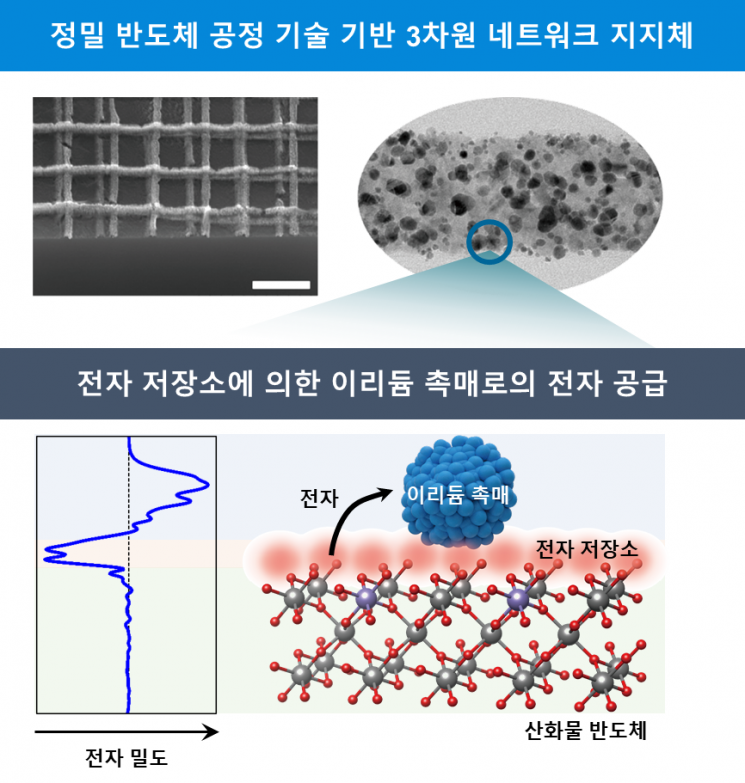 Hydrogen production catalyst using an oxide semiconductor with a three-dimensional network structure containing surplus electron storage as a support. Image source: Provided by KAIST
Hydrogen production catalyst using an oxide semiconductor with a three-dimensional network structure containing surplus electron storage as a support. Image source: Provided by KAIST
KAIST announced on the 25th that Professor Jeong Yeon-sik of the Department of Materials Science and Engineering, in collaboration with Dr. Kim Jin-young and Dr. Kim Dong-hoon from the Korea Institute of Science and Technology (KIST), developed a high-efficiency and high-durability hydrogen production technology by applying a new principle where the hydrogen production catalyst replenishes electrons lost during the reaction from a novel oxide semiconductor.
To produce high-purity green hydrogen, an eco-friendly polymer electrolyte membrane water electrolysis (PEMWE) device powered by renewable energy is used. In this process, iridium (Ir) catalysts, which are mainly used, must continuously maintain a state rich in electrons to achieve both high efficiency and durability. However, due to the nature of catalyst reactions that easily lose electrons and oxidize, there has been a chronic problem of significant degradation in efficiency and lifespan.
The research team utilized semiconductor technology capable of stacking ultrafine patterns to implement a three-dimensional network structure. The material used was tin oxide doped with antimony (Sb), and semiconductor deposition technology was applied so that oxygen ions, which act as an ‘electron reservoir,’ are densely distributed on the surface of this oxide. When this unique oxide semiconductor is used as a catalyst support, the oxygen ions located on the surface continuously supply sufficient electrons to the iridium (Ir) catalyst, thereby maintaining high hydrogen production efficiency of the catalyst over a long period.
When the research team applied this to a polymer electrolyte membrane water electrolysis (PEMWE) device, they achieved a world-class performance improvement up to 75 times better than existing commercial iridium (Ir) nanoparticle catalysts, while also securing excellent durability for long-term operation at high current densities.
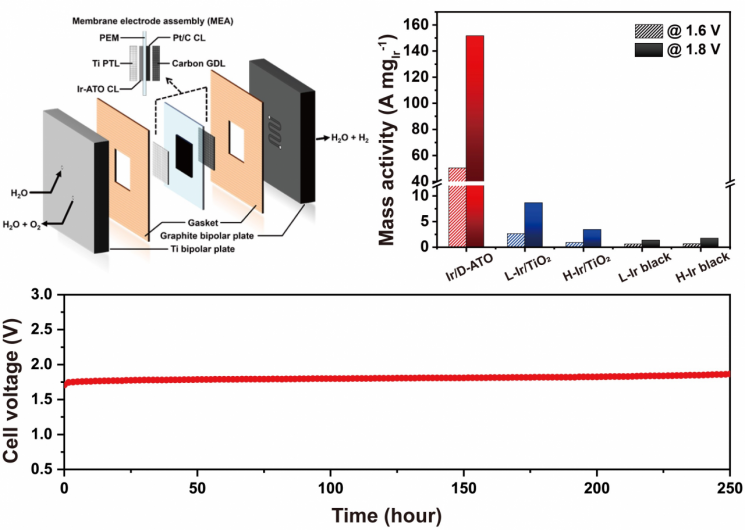 Hydrogen production catalyst performance using an oxide semiconductor containing surplus electron storage as a support. Image source: Provided by KAIST
Hydrogen production catalyst performance using an oxide semiconductor containing surplus electron storage as a support. Image source: Provided by KAIST
Professor Jeong said, “Generally, semiconductor technology and hydrogen production are considered very different fields, but by implementing materials with unique compositions difficult to obtain through conventional synthesis techniques using precise semiconductor processing technology, we were able to achieve high efficiency. This research is a good example demonstrating the importance of convergence between technological fields.” Dr. Kim Jin-young of KIST added, “By using less than one-tenth the amount of precious metal catalysts compared to existing ones while achieving equal or better performance, we expect that further research will secure the economic feasibility of green hydrogen production.”
The research results were published online on the 5th of last month in the international academic journal Nature Communications.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














