Developed as a Type 2 Diabetes Treatment
But Noted for Significant Weight Loss Effect as an Obesity Therapy
Confirmed Cardiovascular Disease Prevention and Type 1 Diabetes Treatment Effects
Attempting Expansion into Dementia and NASH Therapies
Can It Enter Insurance Coverage Through Major Disease Treatments?
Glucagon-like peptide (GLP)-1 drugs, which are rapidly expanding their market as treatments for type 2 diabetes and obesity, are now being explored for various other diseases such as type 1 diabetes, non-alcoholic steatohepatitis (NASH), and Alzheimer's dementia following their cardiovascular benefits. Since these diseases have high unmet needs due to the lack of fundamental treatments, there is growing anticipation that the market could experience explosive growth.
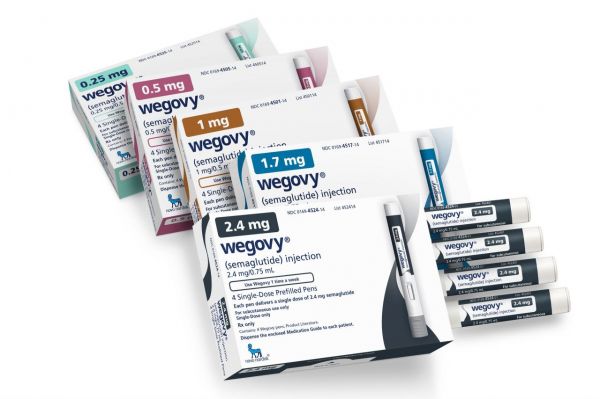 Novo Nordisk's GLP-1 class treatment 'Wegovy (active ingredient Semaglutide)'
Novo Nordisk's GLP-1 class treatment 'Wegovy (active ingredient Semaglutide)' [Photo by Novo Nordisk]
GLP-1 was initially developed as a treatment for type 2 diabetes after its effect of promoting insulin secretion and lowering blood sugar was confirmed. However, as its appetite-suppressing effect and the ability to slow gastric emptying were identified, its indications expanded to obesity, and recently, its cardiovascular disease prevention effects have also been confirmed.
Novo Nordisk, which developed the GLP-1 class drug Wegovy (active ingredient semaglutide), announced last month that in the 'SELECT' clinical trial verifying semaglutide's effect as an adjunct to standard treatment for major adverse cardiovascular events (MACE) prevention, the MACE rate in the treatment group decreased by 20%. Eli Lilly, which developed another GLP-1 class diabetes and obesity drug Mounjaro (tirzepatide), is also planning to confirm whether tirzepatide can reduce deaths caused by cardiovascular disease through the 'SURMOUNT-MM0' trial. The trial began in October last year, with results expected in 2027.
Unlike type 2 diabetes, which is mainly caused by lifestyle factors, there is also analysis suggesting that GLP-1 treatments may be effective for type 1 diabetes, which develops due to genetic issues. Type 1 diabetes is a refractory disease where immune cells attack and destroy pancreatic beta (β) cells, preventing insulin production. There is no fundamental cure, so patients must receive insulin injections for life. Although 'Tzield (teplizumab)', which delays onset, was approved by the U.S. Food and Drug Administration (FDA) last year, it is not a fundamental treatment either.
According to a recent paper published by researchers at the State University of New York at Buffalo in the international journal The New England Journal of Medicine (NEJM), when semaglutide was administered to 10 early-stage type 1 diabetes patients, the key biomarker glycated hemoglobin (HbA1c) levels were maintained without insulin injections. Before treatment, these patients had an average HbA1c level of 11.7%, far above the normal range of 4?6%, and had been continuously receiving basal and mealtime insulin.
However, within three months of semaglutide treatment, they completely stopped mealtime insulin, and seven out of ten patients discontinued basal insulin within six months. Despite this, the average HbA1c level dropped to 5.9% at six months and 5.7% at twelve months, falling within the normal range. The researchers explained that even type 1 diabetes patients retain some insulin reserves, and semaglutide stimulates β cells to secrete this insulin, allowing HbA1c to be maintained without separate insulin injections.
Going a step further, there is also hope that GLP-1 drugs could be used to treat dementia. Novo Nordisk began the phase 3 'EVOKE' clinical trial in May 2021 in South Korea, the United States, and Japan to prove semaglutide's effect on improving Alzheimer's dementia, with completion expected between 2025 and 2026. The trial is also ongoing in South Korea, where Professor Han Seol-hee of Konkuk University Hospital's Neurology Department, the domestic clinical lead, explained, "Both diabetes and Alzheimer's dementia involve brain inflammation as a key cause, so the idea started from the possibility of eliminating the toxicity of amyloid beta (Aβ), a major cause of dementia, through this. We expect to be able to remove oxidative stress and neuroinflammation that occur after significant Aβ accumulation."
The significance of expanding to other diseases lies in its potential to open the door to insurance coverage in the future. Currently, in the United States, most insurers do not cover obesity treatments, and Medicare, the public insurance, legally cannot cover them. However, if effects on cardiovascular disease, NASH, dementia, and other obesity-related complications are confirmed, the barrier to insurance coverage could be easily overcome, and demand is expected to increase exponentially.
In South Korea, development for indications other than type 2 diabetes and obesity is mainly focused on NASH treatment. Although it is a disease often called the "big pharma graveyard" due to repeated failures by global big pharmaceutical companies, recent progress has raised expectations as Madrigal Pharmaceuticals' 'Resmetirom' has entered the FDA approval process.
Hanmi Pharmaceutical, led by Lim Joo-hyun, Head of Strategic Planning at Hanmi Science (President of Hanmi Pharmaceutical), is advancing the 'H.O.P (Hanmi Obesity Pipeline)' project aiming to launch a GLP-1-based obesity drug tailored for Koreans by 2025. Hanmi is leading this field with dual agonist 'Epinofepegdutide', which combines GLP-1 and glucagon (GCG) to increase energy metabolism, licensed out to MSD (Merck & Co., USA) and currently in phase 2b clinical trials. Additionally, the triple agonist 'Efocipegtrutide (lab triple agonist)', which also targets the glucose-dependent insulinotropic polypeptide (GIP) receptor to stimulate insulin secretion, is in phase 2 trials.
Dong-A ST plans to submit an Investigational New Drug (IND) application for 'DA-1276', a GLP-1 and glucagon dual agonist NASH and obesity treatment licensed to its U.S. subsidiary NeuroBo Pharmaceuticals, by the second half of this year. The G protein-coupled receptor 119 (GPR119) agonist mechanism that increases GLP-1, 'DA-1241', a NASH and type 2 diabetes treatment, is currently in phase 2 trials and recently began dosing the first patient. 'YH25724', licensed by Yuhan Corporation to Boehringer Ingelheim, also recently started phase 1 trials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![From Hostess to Organ Seller to High Society... The Grotesque Scam of a "Human Counterfeit" Shaking the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















