The molecular mechanism of how Tau protein, a major cause of Alzheimer's dementia, aggregates and fibrillates in the brain has been demonstrated for the first time in the world by a domestic research team.
 Professor Minjae Lee, Department of Medical Science, Seoul National University College of Medicine [Photo by Korea Health Industry Development Institute]
Professor Minjae Lee, Department of Medical Science, Seoul National University College of Medicine [Photo by Korea Health Industry Development Institute]
The Korea Health Industry Development Institute announced on the 4th that a multidisciplinary research team led by Professor Minjae Lee of the Department of Medical Science at Seoul National University College of Medicine and a research team led by Professor Jungon Kim of the Department of Chemistry at Korea University have for the first time demonstrated the fibrillation process of Tau protein and the principle of neurotoxic substance formation.
Alzheimer's dementia, which is known to account for 70-80% of dementia patients, has been considered primarily caused by the accumulation of proteins such as amyloid beta (Aβ) and Tau in the brain, forming toxic substances that kill nerve cells. However, the more fundamental pathogenic mechanisms, such as why these proteins accumulate, have not yet been elucidated, posing an obstacle to the development of disease-modifying therapies (DMTs).
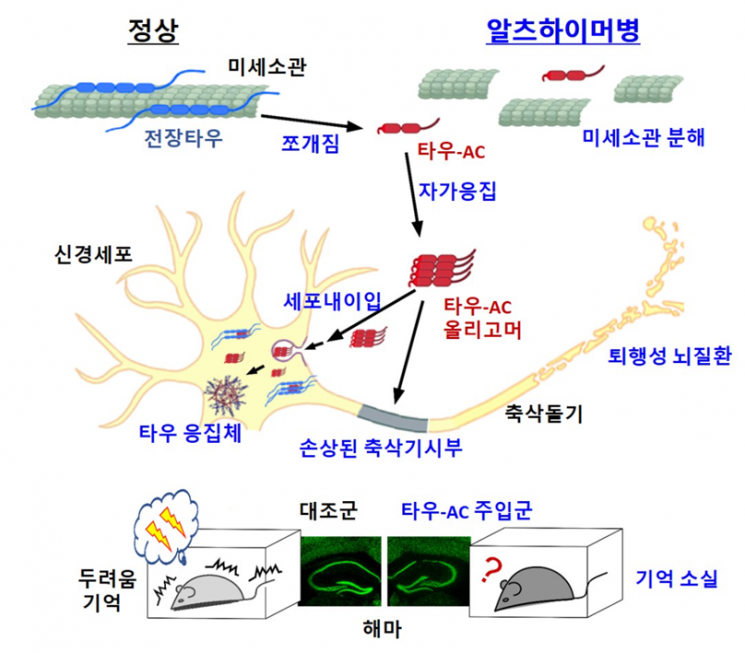
This study specifically elucidated the previously unknown fibrillation phenomenon of Tau protein, including the process by which Tau protein fragments penetrate inside brain nerve cells, the mechanism of synaptic function inhibition, and the effects on memory decline and brain tissue necrosis in animals.
To understand how Tau protein fibrillates at the molecular level, the researchers focused on the core aggregation region that promotes the formation of neurotoxic substances. As a result, they confirmed that a portion of internally cleaved Tau protein can spontaneously form neurotoxic substances under physiological environmental conditions without additional treatment and that it has the ability to convert normal Tau protein into neurotoxic substances. They also confirmed that this is because the cleavage exposes a specific site (aggregation core, Tau-AC) of the protein.
Additionally, they identified the pathway by which neurotoxic substances generated by Tau cleavage penetrate nerve cells, the process inducing further aggregation, and the phenomenon of reducing synaptic plasticity in nerve cells, thereby establishing the pathological induction mechanism at the cellular level.
Moreover, in animal model experiments, when the Tau aggregation core was injected into the hippocampus of mouse brain ventricles, they observed behavioral changes similar to Alzheimer's dementia, such as nerve cell death, neuroinflammatory responses, and memory decline, confirming that the pathological mechanism of Tau fragments elucidated at the molecular and cellular levels was reproduced in the animal model.
 Alzheimer's Disease Onset Model Starting from Tau Cleavage
Alzheimer's Disease Onset Model Starting from Tau Cleavage [Photo by Korea Health Industry Development Institute]
Professor Minjae Lee, who led the research, explained the significance of the study, saying, "We have revealed the new principles of Tau protein fibrillation and neurotoxic substance generation at the molecular, cellular, and animal model levels," and added, "Through follow-up research, this will contribute to proposing new treatment methods for Alzheimer's disease." He also emphasized the need for continuous government support for creative research and development (R&D) activities, stating, "The research achievements were produced through collaboration among researchers in various fields such as biochemistry, neuroscience, and biophysics, and national support for high-risk, high-reward basic research."
This research was conducted with the support of the Dementia Overcoming Research and Development Project Group. It was published online on the 18th of last month in the international academic journal Advanced Science.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















