First Hurdle Passed by Cancer Disease Review Committee
Competitor AZ's 'Tagrisso' on the Same Level
Expanded Coverage Expected Within the Year at the Earliest
Yuhan Corporation's lung cancer new drug 'Reclaza' (active ingredient: Lazertinib) is showing rapid progress in expanding first-line treatment coverage. With the possibility of coverage expansion as early as this year, Reclaza has passed the first hurdle?the Cancer Disease Review Committee?at once, a milestone that took the competing drug AstraZeneca (AZ)'s 'Tagrisso' (Osimertinib) a full five years to overcome, placing Reclaza now on the same level as Tagrisso.
 Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (active ingredient: Lazertinib)'
Yuhan Corporation's non-small cell lung cancer treatment 'Leclaza (active ingredient: Lazertinib)' [Photo by Yuhan Corporation]
The Health Insurance Review and Assessment Service (HIRA) announced on the 30th that at the 6th Cancer Disease Review Committee meeting in 2023, it set the reimbursement criteria to expand coverage for Reclaza as a first-line treatment for cancer patients.
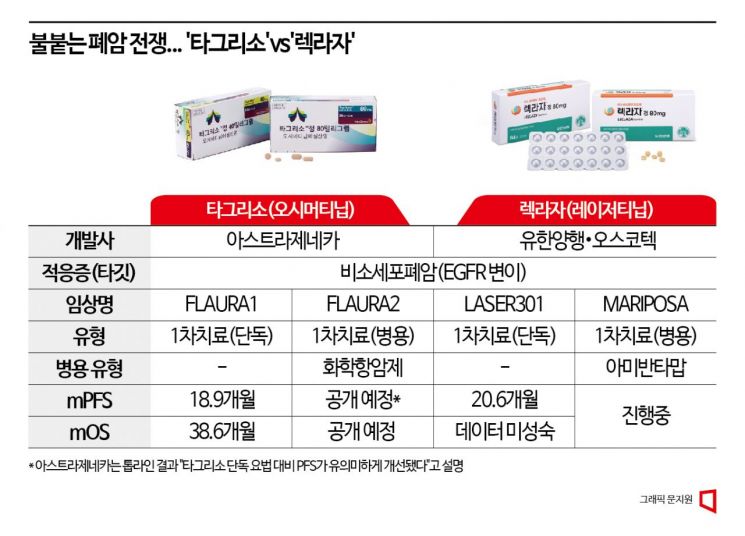
Developed by Yuhan Corporation and Oscotec, Reclaza was approved in January 2021 as Korea's 31st new drug for second-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) T790M mutation. In June of this year, its indication was successfully expanded to first-line treatment for patients with locally advanced or metastatic NSCLC harboring EGFR exon 19 deletion or exon 21 (L858R) substitution mutations. Subsequently, in just over two months, it took the first concrete step toward expanding national health insurance coverage to include first-line treatment.
In contrast, Tagrisso, despite receiving early approval from the Ministry of Food and Drug Safety (MFDS) as a first-line treatment in 2018, only succeeded in passing the Cancer Disease Review Committee after five attempts in March of this year. For anticancer drugs, reimbursement requires passing not only the Cancer Disease Review Committee but also the Drug Reimbursement Evaluation Committee and the Health Insurance Policy Review Committee. Tagrisso has yet to pass the Drug Reimbursement Evaluation Committee since March, effectively placing both drugs at the same starting line after overcoming a five-year gap.
As Tagrisso's reimbursement application within this year becomes increasingly uncertain, Yuhan Corporation is currently conducting an Expanded Access Program (EAP) for Reclaza's first-line treatment. This program provides Reclaza free of charge to patients eligible for first-line treatment, with the drug priced at 75.5 million KRW annually based on the insurance price for second-line treatment. Since there are currently no third-generation EGFR tyrosine kinase inhibitors (TKIs) covered for first-line treatment, Yuhan plans to continue this free support in the spirit of the late doctor's social contribution. The first patient was registered last month in Busan, and actual support has begun.
Reclaza is not stopping there; it is conducting the 'MARIPOSA' combination clinical trial with Janssen's NSCLC treatment 'Rybrevant' (Amivantamab). The results are expected to be unveiled at the European Society for Medical Oncology (ESMO), one of the world's top three oncology conferences, held in Madrid, Spain, this October. Reclaza monotherapy has already demonstrated a median progression-free survival (mPFS) of 20.6 months, surpassing Tagrisso, and the goal is to secure even better results through combination therapy to decisively gain the upper hand in the first-line treatment battle. However, Rybrevant, which applied for reimbursement for the indication of "treatment of locally advanced or metastatic NSCLC patients with EGFR exon 20 insertion mutation whose disease progressed during or after platinum-based chemotherapy," failed to pass as reimbursement criteria were not established at the Cancer Disease Review Committee.
Earlier, AZ also announced that it will reveal the clinical trial results of 'FLAURA2,' which combines Tagrisso with platinum-based chemotherapy agents such as cisplatin as a first-line treatment for NSCLC patients with EGFR mutations, at the 2023 World Conference on Lung Cancer (WCLC) held in Singapore from the 9th to 12th of next month (local time). The presentation is scheduled for the plenary session on the 11th.
Meanwhile, at the same Cancer Disease Review Committee meeting, Takeda's 'Exkivity' (Mobocertinib), which applied for reimbursement for the same indication as Rybrevant, also failed to establish reimbursement criteria. On the other hand, Korean Roche's metastatic and early breast cancer treatment 'Pessco' (Pertuzumab and Trastuzumab), Pfizer's NSCLC treatment 'Lorbrena' (Lorlatinib), and Merck's colorectal cancer treatment 'Erbitux' (Cetuximab) all passed their requested reimbursement decisions and expansions.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.