UNIST Professor Tae-Eun Park's Team Develops Microfluidic-Based Gastric Mucosal Barrier Mimicking Biotech Chip
Opening Path to Reproduce Helicobacter Infection Situations... Foundation for Gastric Cancer and Gastrointestinal Disease Research
A domestic research team has opened the way to defend against Helicobacter bacteria by developing a biochip that mimics the human gastric mucosal system.
This is evaluated as laying the foundation for studying various gastrointestinal diseases, including gastric cancer, by reproducing the infection situation of Helicobacter bacteria.
UNIST (President Yong-Hoon Lee) announced on the 28th that Professor Tae-Eun Park's research team from the Department of Biomedical Engineering and Professor Sung-Ho Kong's research team from Seoul National University Hospital (Director Young-Tae Kim) developed a biomimetic chip that mimics the protective function of the actual human gastric mucosa by combining organoid and organ-on-a-chip technologies.
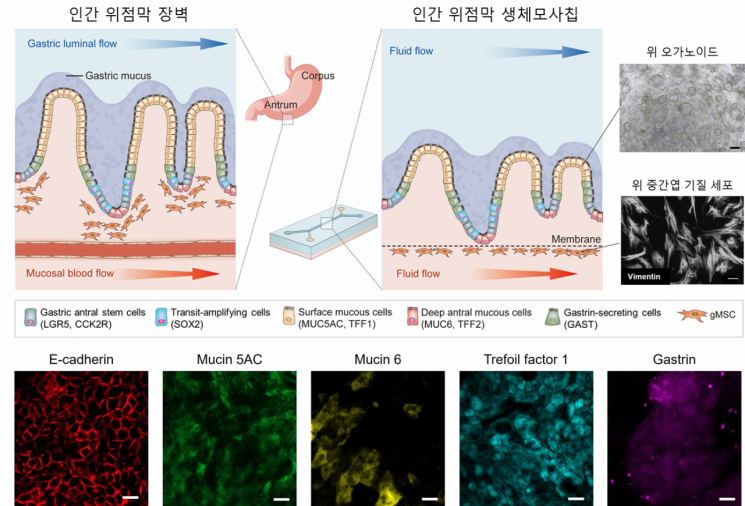 Schematic diagram of the human stomach microphysiological system (human stomach MPS) and the conceptual diagram confirming the stomach epithelial cell-specific expression cells.
Schematic diagram of the human stomach microphysiological system (human stomach MPS) and the conceptual diagram confirming the stomach epithelial cell-specific expression cells.
Organoids are organ-like structures created from stem cells that replicate human organs. They are attracting attention as in vitro models that can replace animal experiments by better mimicking specific functions than traditional culture methods. However, they have limitations in replicating mechanical stimuli and intercellular interactions of the human body.
The research team succeeded in developing a biochip that implements a gastric mucosa similar to the real one and mimics its defense mechanism characteristics. Mechanical stimuli were simulated through microfluidic flow within the biochip, facilitating smooth intercellular interaction flows.
According to the research team, mesenchymal stromal cells exposed to fluid flow activate the proliferation of gastric stem cells and maintain the balance of differentiation. Ultimately, this biomimics the proportion and maturity of gastric mucus-secreting cells necessary for developing the gastric mucosal barrier at a biological level.
In the developed biochip, the research team discovered one of the defense mechanisms of Helicobacter bacteria that could not be observed in existing models. The gastric mucosal peptide (Trefoil factor 1, TFF1) provides stability to the gastric mucosal layer and protects gastric epithelial cells from external infectious agents.
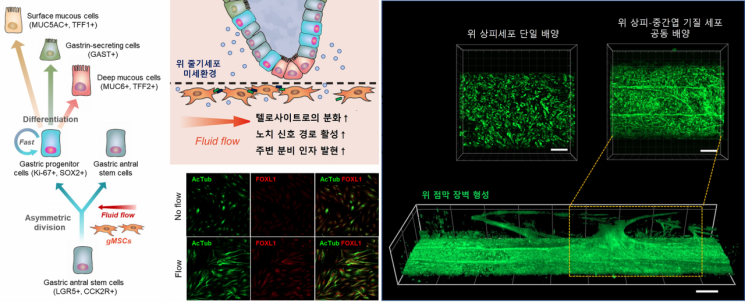 Study on the maintenance of gastric epithelial cell homeostasis (regulation of stem cell capacity and differentiation capacity) and gastric mucosal barrier formation through epithelial-mesenchymal stromal cell interactions within the gastric mucosa biomimetic chip.
Study on the maintenance of gastric epithelial cell homeostasis (regulation of stem cell capacity and differentiation capacity) and gastric mucosal barrier formation through epithelial-mesenchymal stromal cell interactions within the gastric mucosa biomimetic chip.
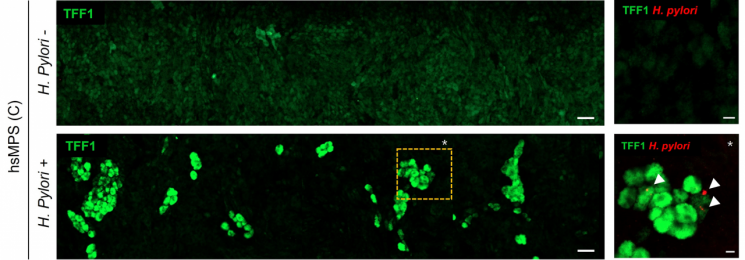 A study on the human gastric mucosal barrier biomimetic chip used as a Helicobacter pylori infection model and changes in the expression pattern of gastric mucosal peptide (TFF1).
A study on the human gastric mucosal barrier biomimetic chip used as a Helicobacter pylori infection model and changes in the expression pattern of gastric mucosal peptide (TFF1).
The research team observed for the first time in the world that the gastric mucosal peptide is expressed in a mosaic pattern around clusters of Helicobacter bacteria, forming a fence and establishing an efficient defense system.
Conversely, when the expression of the gastric mucosal peptide was suppressed, more severe inflammatory responses were confirmed.
Professor Tae-Eun Park of the Department of Biomedical Engineering said, “This presents the possibility as a model that can observe the dynamic inflammatory system interactions between epithelial cells and immune cells in chips actually infected with Helicobacter pylori.”
 From the top row, left: Researcher Jonathan Sabat del R?o, Professor Kang Jooheon; from the bottom row, left: First author Researcher Jeong Hyejin, Corresponding author Professor Park Tae-eun.
From the top row, left: Researcher Jonathan Sabat del R?o, Professor Kang Jooheon; from the bottom row, left: First author Researcher Jeong Hyejin, Corresponding author Professor Park Tae-eun.
First author researcher Hye-Jin Jung explained, “This can contribute to a comprehensive understanding of the stability of the gastric mucosal barrier and serve as a cornerstone for developing in vitro gastrointestinal models that can replace animal experiments.”
She also said, “As the first human gastrointestinal biomimetic chip capable of reproducing the in vivo environment of the gastric mucosal barrier, it is expected to improve drug development and therapeutic accessibility for infectious agents such as bacteria and viruses in the stomach.”
This research was conducted under the Basic Research Laboratory Support Project and the Excellent Young Researcher Project of the National Research Foundation of Korea, Ministry of Science and ICT, and was published online on July 31 in Advanced Science, a world-renowned academic journal published by Wiley.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















