UNIST Professor Hyunwook Lee's Team Publishes Two Consecutive Papers on New Materials
Energy Density Over 1.3 Times Higher Than Vanadium Electrolyte
New research enhancing the performance of the ‘redox flow battery’ system, which is safer than lithium-ion batteries and made from inexpensive materials, is drawing global academic attention.
A material that dramatically improves the performance of redox flow batteries has been announced by a domestic research team, expected to lay the foundation for the development of new materials.
The research teams led by Professor Hyunwook Lee from the Department of Energy and Chemical Engineering at Ulsan National Institute of Science and Technology (UNIST, President Yong-Hoon Lee) and Professor Donghwa Seo from KAIST announced on the 22nd that they have developed a new redox flow battery.
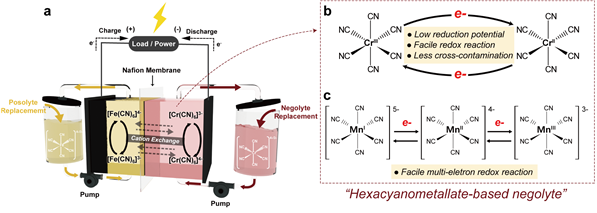 (a) Schematic diagram of the novel iron-chromium redox flow battery designed in this study. (b), (c) Characteristics and advantages of the compounds used as anode electrolytes.
(a) Schematic diagram of the novel iron-chromium redox flow battery designed in this study. (b), (c) Characteristics and advantages of the compounds used as anode electrolytes.
The research team significantly improved the performance of redox flow batteries by attaching stable ligands to redox-active materials. The materials proposed by the team are iron-chromium and iron-manganese redox flow batteries, which are cheaper and made from more abundant elements compared to conventional vanadium.
Redox flow batteries are systems that store energy through oxidation-reduction reactions occurring in active materials that generate energy at the cathode and anode of the battery. They are gaining attention as safer batteries with a lower risk of explosion compared to lithium-ion batteries.
However, vanadium, which is a key component in redox flow batteries, has limited deposits concentrated in specific countries, leading to high price volatility. Additionally, low operating voltage and slow redox reaction rates have posed limitations on improving battery performance.
The research team utilized ‘hexacyanometallates,’ octahedral-shaped complexes where six cyanide ligands (CN-) are attached to transition metal ions such as iron, chromium, and manganese.
Due to their excellent electrochemical properties, the team proposed using hexacyanometallates as the anode electrolyte for the first time in the world, significantly improving stability issues.
Professor Hyunwook Lee of the Department of Energy and Chemical Engineering explained, “We have continuously considered using redox flow batteries as energy storage devices, but the high cost of vanadium posed challenges for scaling up. This research has somewhat solved the challenges of redox flow batteries by developing affordable and high-performance materials.”
The research team operated two types of redox flow batteries using hexacyanometallates: iron-chromium and iron-manganese. In the case of the iron-chromium redox flow battery, it maintained a high coulombic efficiency of over 99% even after more than 500 repeated charge-discharge cycles. It achieved a high voltage of over 1.5V, demonstrating an energy density (38.6 W L-1) more than 1.3 times higher than conventional redox flow batteries.
In the iron-manganese redox flow battery, hexacyanomanganate is used as the anode electrolyte. This enables a ‘two-electron reaction’ that undergoes redox reactions twice, allowing for twice the energy density compared to the same concentration.
Using Raman spectroscopy, which analyzes molecular vibrations by irradiating the material with ultraviolet light, the team confirmed that the two-electron reaction proceeded smoothly even during the charge-discharge process. Stability was also verified through more than 100 repeated charge-discharge cycles.
Jieun Jang, the first author and a combined master's and doctoral course researcher, stated, “This study demonstrated the best performance among chromium-based new materials reported so far for redox flow batteries. By proposing materials capable of fast redox reactions and two-electron reactions, it will greatly contribute to securing diversity in redox flow battery systems.”
This research was conducted with support from Ulsan National Institute of Science and Technology, the Ministry of Science and ICT’s National Research Foundation Individual Research Project, and the Korea Energy Technology Evaluation and Planning’s Energy Human Resources Development Project. The research results were published online on July 7 and August 8 in the renowned international energy journals Advanced Energy Materials and ACS Energy Letters, respectively.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















