A study has revealed that the global cell and gene therapy (CGT) market could reach up to 54 trillion KRW by 2027. Alongside technological development, policy support such as regulatory improvements is also necessary for the growth of the domestic CGT industry.
The National Biotechnology Policy Research Center recently published a report titled "Global Cell and Gene Therapy (CGT) Market Outlook" containing these findings. According to the report, CGT is a new therapeutic modality categorized into cell therapy, gene therapy, and genetically modified cell therapy.
Cell therapy refers to pharmaceuticals created by manipulating cells, such as culturing or selecting them. Gene therapy fundamentally treats intractable diseases by correcting genetic defects or suppressing/amplifying gene functions. Gene therapy is divided into viral vectors and non-viral vectors depending on whether viruses are used to deliver genetic material. Genetically modified cell therapy involves inducing genetic modifications in cells outside the body and transplanting them into patients; therapeutic genes are introduced and cultured in cells ex vivo, then injected into the body using carriers.
CGT is considered one of the promising technologies in the bio industry. The second-generation modality that led the market, antibody drugs, used recombinant DNA and monoclonal antibodies for targeted therapy but had limitations targeting inside cells due to their large molecular weight. Therefore, the center explains that CGT, a third-generation modality capable of gene-level treatment, is gaining attention. Compared to existing therapies, CGT offers fundamental treatment possibilities, raising expectations for treating not only cancer but also neurodegenerative and genetic diseases. The center assessed that "(CGT) is expected to be a next-generation therapy that addresses unmet needs in the market and enables personalized precision treatment."
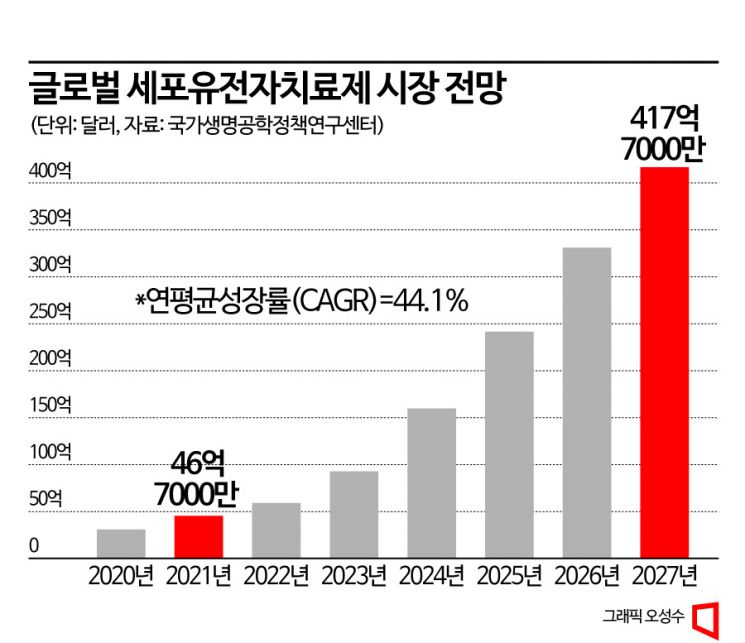
The global CGT market size is also expected to grow rapidly every year. According to market research firm Frost & Sullivan, the global CGT market is projected to expand from $4.67 billion (approximately 6.06 trillion KRW) in 2021 to $41.77 billion (approximately 54.17 trillion KRW) by 2027, growing about ninefold in seven years. The compound annual growth rate (CAGR) during this period is expected to reach 44.1%.
With the promising technology and high market growth prospects, global big pharma companies are also entering CGT research. So far, companies holding chimeric antigen receptor (CAR)-T therapies, a type of CGT, have recorded significant sales. Novartis launched 'Kymriah,' a CAR-T cell therapy for acute lymphoblastic leukemia, followed by 'Zolgensma,' a gene therapy for spinal muscular atrophy (SMA). Gilead Sciences introduced 'Yescarta' for relapsed/refractory large B-cell lymphoma and 'Tecartus' for acute leukemia. Bristol Myers Squibb (BMS) also received approval for its CAR-T cell therapy 'Breyanzi' as a second-line treatment for hematologic cancers.
Domestic companies are primarily entering the CGT market through contract development and manufacturing organizations (CDMO). Samsung Biologics announced at Bio USA last month that it will establish a 'Multi Modal' production facility, including CGT, on the site of its 5th plant in Songdo, Incheon. SK Bioscience also announced a five-year growth plan to promote CDMO for new platforms including CGT. Additionally, SK Inc.'s CDMO subsidiary SK Pharmteco completed a local CGT factory through its French subsidiary Iposketch, and CJ (Batavia) and GC Cell (BioCentric) are preparing for CGT contract manufacturing by acquiring CDMO companies.
To foster the growth of the CGT market, technological development must be supported by policy measures. The National Biotechnology Policy Research Center suggested, "To promote future growth of the CGT market, it is necessary to develop high-quality, low-cost CGT through technological development and manufacturing capacity enhancement," adding, "Policy support such as drug pricing systems and regulatory improvements is also required."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















