Domestic Researchers Develop Intranasal Vaccine
Domestic researchers have developed a vaccine that can block the COVID-19 virus in the nose.
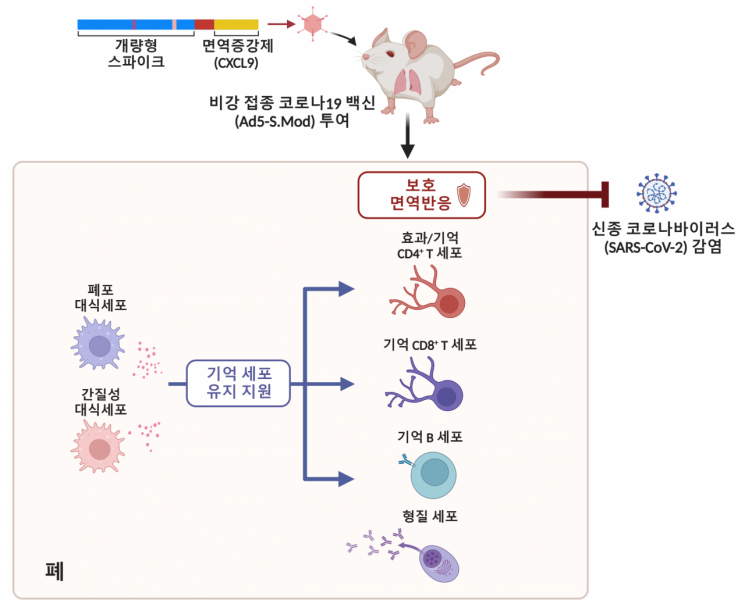 A schematic summary of a nasal vaccination research model that blocks the COVID-19 virus from the nostrils. Image source: Provided by KAIST
A schematic summary of a nasal vaccination research model that blocks the COVID-19 virus from the nostrils. Image source: Provided by KAIST
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 29th that a research team led by Professor Lee Heung-gyu of the Graduate School of Medical Science and Engineering, in collaboration with the Korea Research Institute of Chemical Technology, has developed a nasal COVID-19 vaccine using an adenovirus vector platform. The team also confirmed through mouse models that this vaccine induces a strong mucosal immunity that lasts for a long time.
Although the COVID-19 pandemic emergency was lifted after 3 years and 4 months, variants caused by frequent mutations continue to be reported, leaving the possibility of a resurgence. These variants are evolving to evade the existing immune responses acquired by hosts through vaccination or infection. Since currently available intramuscular vaccines have limitations in sufficiently inducing mucosal immunity that can suppress viral spread, there remains a need for vaccines that can induce strong and long-lasting mucosal immunity.
Mucosal immunity refers to the immune response that acts on mucous membranes, which are epithelial tissues forming the respiratory, digestive, and reproductive tracts. It is a defense system that recognizes and eliminates external agents invading the mucosa, which serves as the interface between the inside and outside of the body, thereby protecting the human body from infection. Because mucosa is a major entry route for pathogens or external antigens, mucosal immunity has the advantage of responding more rapidly to pathogen invasion than systemic immune responses, thereby preventing infection and spread.
The research team developed a nasal COVID-19 vaccine (Ad5-S.Mod) based on an adenovirus vector platform, carrying a modified spike protein antigen and a human-derived immune adjuvant (CXCL9), and verified its efficacy using experimental mice. They focused on the fact that the human-derived immune adjuvant attracts activated T cells to specific locations, using it as an immune enhancer to improve the vaccine's effectiveness.
The team tested the vaccine's efficacy with a single nasal administration or a two-dose regimen of intramuscular followed by nasal administration. As a result, they confirmed that the nasal vaccine could induce high levels of antibody responses and memory T cell responses in the respiratory mucosa. The vaccinated group showed 100% survival upon infection with the novel coronavirus with just a single dose. Furthermore, they demonstrated that the immune response induced by the vaccine was maintained for at least one year, proving the vaccine's strong ability to induce mucosal immunity.
Using single-cell transcriptome analysis, the researchers also confirmed that lung macrophages in vaccinated experimental mice are the main producers of factors that help maintain memory T cells and B cells, and that removal of lung macrophages significantly reduces memory T and B cells in the lungs. This clarified that these cells support the maintenance of respiratory memory cells generated by the mucosal vaccine.
This study presents a new vaccine design strategy that enhances vaccine efficacy by utilizing the human-derived immune adjuvant (CXCL9). It newly identifies the important role of lung macrophages in maintaining memory immune responses formed by mucosal vaccines, suggesting a new target that can be used for developing nasal vaccines against various pathogens.
The results of this study were published online on the 14th in the international virology journal 'Antiviral Research.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















