FDA Approval for Alopecia Areata Treatment 'Litpulo'
Clinical Results Show 1 in 4 Patients Regain 80% Hair Restoration
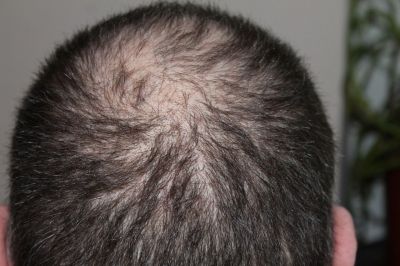
Pharmaceutical company Pfizer's alopecia areata treatment 'Litfulo' (Litfulo, Littlelecitinib) has been approved by the U.S. Food and Drug Administration (FDA).
On the 23rd (local time), Reuters reported that with the FDA's approval, adolescents aged 12 and older suffering from severe hair loss can now use Litfulo as a treatment. Litfulo is recommended to be taken orally once daily at a dose of 50mg.
Litfulo belongs to the Janus kinase (JAK) inhibitor class used to treat inflammatory diseases. Unlike common hair loss, alopecia areata occurs when the immune system attacks hair follicles, causing hair to fall out. JAK inhibitors treat alopecia areata by blocking immune activation pathways. Previously, in June last year, Eli Lilly's 'Olumiant' was the first in this inhibitor class to receive FDA approval, and Litfulo is the second.
In a clinical trial involving 718 patients with hair loss symptoms on more than 50% of the scalp, 23% of those who took 50mg of Litfulo showed hair growth on more than 80% of the scalp after six months. Additionally, the efficacy and safety of Litfulo were consistent in both adolescents (ages 12?17) and adults (18 and older). However, at least 4% of subjects taking Litfulo experienced side effects such as headaches, diarrhea, acne, rashes, and hives.
Brett King, Associate Professor of Dermatology at Yale School of Medicine, stated, "Alopecia areata affects everyone and is not uncommon among young populations, including adolescents. Having an approved drug treatment for adolescents is an important leap forward."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















