Professor Jeon Gijun of Inha University and Professor Park Cheolmin of Kumoh National Institute of Technology Research Team
Platinum-Equivalent Efficiency and 1000 Times Higher Catalyst Mass Activity
Water electrolysis technology, which obtains hydrogen and oxygen by electrolyzing water, is one of the core technologies of renewable energy. However, existing technologies have the disadvantage of high costs due to high electricity consumption and the need for expensive platinum catalysts. A domestic research team has developed a technology that can achieve similar efficiency using inexpensive molybdenum disulfide instead of platinum, significantly reducing costs.
The National Research Foundation of Korea announced on the 13th that the research team led by Professor Jun Ha Na from Inha University and Professor Cheolmin Park from Kumoh National Institute of Technology succeeded in developing a technology to control the structure of molybdenum disulfide (MoS2), a two-dimensional material widely used as a catalyst in water electrolysis reactions.
Catalysts are used in the water electrolysis process to increase reaction rates and efficiency. Among catalysts, precious metals with the highest reactivity are expensive, prompting active research to improve economic feasibility. Among these, two-dimensional materials, which are considered excellent catalyst candidates, exhibit higher efficiency in single atomic layers than in multilayers, but even within single layers, performance varies depending on two structurally different phases. There had been limitations in controlling these two different phases while synthesizing high-quality, uniform single layers.
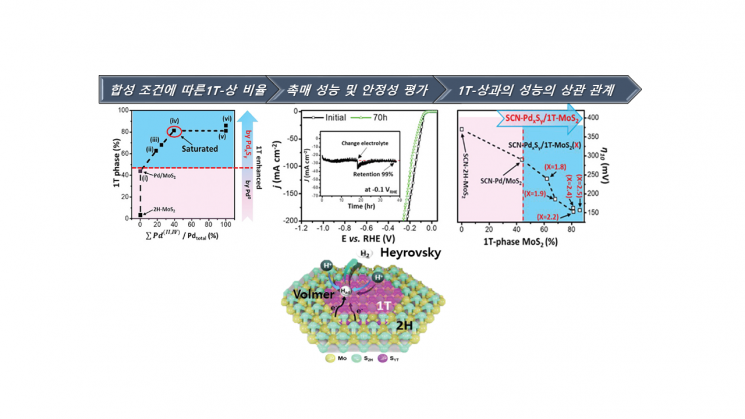
The research team fabricated uniform single atomic layers of molybdenum disulfide using chemical vapor deposition (CVD) and succeeded in controlling the two phases present in the atomic layer by inserting an ultra-small amount (less than 1%) of palladium metal. CVD is a thin-film formation method in which volatile components of materials chemically react with different component gases to form non-volatile solid thin films on a substrate.
The synthesized molybdenum disulfide showed higher purity and water electrolysis efficiency compared to previous synthesis studies. The structural phase transition ratio was controlled up to 86%, achieving a world-class technological level. This is also significant because the team proposed a new standard for confirming structural phase transitions through Raman Spectroscopy, an indicator used to verify this. Raman spectroscopy is an instrument that identifies molecular types by irradiating molecules with a laser and analyzing the energy level differences of electrons. In particular, the hydrogen generation efficiency was comparable to that of the highest-level platinum catalysts, and the catalyst mass activity was confirmed to be more than 1,000 times higher than that of conventional platinum catalysts.
The research team stated, “Through structural control of phases in water electrolysis reaction catalysts, semiconductors, photonic devices, and electronic devices, it will be possible to strategically adjust the phase ratio suitable for each field, enabling various research and industrial applications.”
The research results were published on April 25 in the international energy journal Advanced Energy Materials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.