Rapid Increase in US Consumers Taking Semaglutide Raw Material
FDA Warns "No Review of Safety and Quality Conducted"
As more consumers in the U.S. market purchase raw ingredients instead of obesity medications, the U.S. Food and Drug Administration (FDA) has issued warnings about the risks of arbitrary consumption and is taking measures to address the issue.
On the 1st (local time), The Wall Street Journal (WSJ) reported that the FDA has received reports of problems from people who took semaglutide compounds.
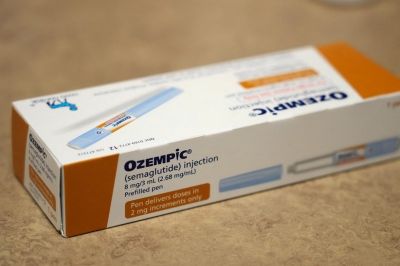
Semaglutide is a drug approved by the FDA for diabetes and obesity treatment and is used as the raw ingredient in diabetes medications such as Wegovy and Ozempic.
Recently, sales of Wegovy and Ozempic surged after it became known that they induce a feeling of fullness and are effective for weight loss.
In particular, a shortage has occurred to the extent that it is difficult to obtain the drugs even with a prescription, as Tesla CEO Elon Musk and model Kim Kardashian revealed that they were prescribed Wegovy and lost weight.
As a result, consumers ordered and took semaglutide salts from pharmacies and online instead of Wegovy and Ozempic, and some problems reportedly occurred.
The FDA did not disclose specific details about the safety issues or the number of reports.
However, the FDA cautioned, "While semaglutide has been approved (for diabetes and obesity treatment), the safety and quality of semaglutide compounds have not been reviewed."
Pharmaceutical company Novo Nordisk, which manufactures Ozempic and Wegovy, also stated that the semaglutide used in compounds differs in terms of safety and quality from that used in drug manufacturing, and taking semaglutide compounds may cause health problems.
In the U.S., pharmacists can sell raw ingredients to consumers if certain medications are unavailable due to shortages.
However, such raw ingredients must have their safety confirmed in advance.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















