GIST Develops High-Power, High-Capacity Redox Battery
Stores and Releases Electricity Using Oxidation-Reduction Reactions
Affordable, Long Lifespan, and High Safety
Domestic researchers have succeeded in developing a next-generation redox battery with high capacity and high output to replace lithium-ion batteries, which have low storage capacity and explosion risks.
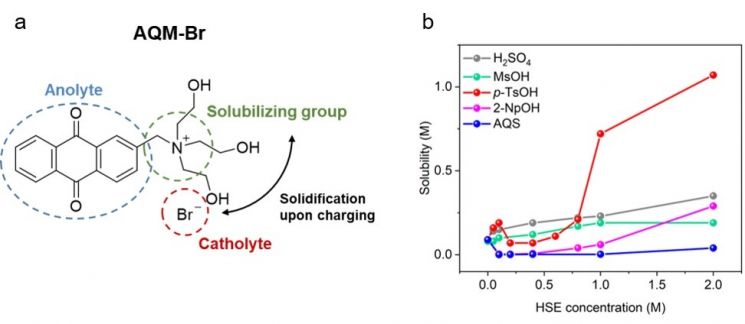 a. Newly developed electrochemically active (redox-active) organic molecule optimized for HSE, 2-[N,N,N-tris(2hydroxyethyl)]anthracenemethanaminum-9,10-dione bromide (AQM-Br)
a. Newly developed electrochemically active (redox-active) organic molecule optimized for HSE, 2-[N,N,N-tris(2hydroxyethyl)]anthracenemethanaminum-9,10-dione bromide (AQM-Br)b. Graph showing the solubility changes of AQM-Br at different concentrations in each HSE solution. A sharp increase in solubility can be observed when using p-TsOH electrolyte.
The Gwangju Institute of Science and Technology (GIST) announced on the 16th that a joint research team led by Professors Seungjun Yoo and Seokwon Hong developed an electrolyte that significantly enhances the performance of redox-active organic molecules, successfully realizing a high-capacity, high-output redox battery.
Lithium-ion batteries, widely used in today's energy storage systems (ESS), have a short lifespan and pose explosion risks due to battery overheating. Various alternatives are being researched, among which the relatively inexpensive, long-lasting, and low-explosion-risk "redox battery" stands out. Redox is a portmanteau of reduction and oxidation. A redox battery refers to a battery in which active materials in the electrolyte undergo redox reactions to store and release electricity as chemical energy in the electrolyte. Since it uses an aqueous (water-based) electrolyte, it fundamentally prevents fires caused by battery overheating. It is a hybrid battery that maximizes capacity by combining the energy storage and release mechanism of redox reactions in secondary batteries with the high output capability of supercapacitors.
By using the "hydrotrope-supported electrolyte" developed by the research team, the solubility of organic molecules used in redox batteries is greatly improved to about six times that of conventional levels, enabling the development of high-capacity redox batteries. To increase the energy capacity of electrochemical-based ESS such as redox batteries, a high concentration of active materials must be dissolved in the electrolyte. However, there have been issues where the solubility of active materials is low, or the addition of supporting electrolytes to increase ionic conductivity reduces solubility.
The research team, inspired by the concept of "hydrotropes"?substances that help materials that do not easily bond with water dissolve well in water?developed molecules that have a hydrotrope structure and act as supporting electrolytes. This supporting electrolyte simultaneously improves the solubility of active materials and ionic conductivity. Hydrotropes are low-molecular-weight amphiphilic substances that are compatible with both water and oil, and unlike conventional surfactants, they are known to enhance the aqueous solubility of poorly water-soluble substances through irregular nanostructures.
Additionally, the research team designed redox-active organic molecules optimized for this supporting electrolyte, enabling stable operation of the battery even in high-concentration electrolytes. The active organic molecules operated stably even in a high-concentration (0.5 Mol/L) electrolyte. This is the highest concentration among quinone-based redox batteries developed to date.
For the first time, nuclear magnetic resonance measurement using the nuclear Overhauser effect was introduced to elucidate the physicochemical principles by which hydrotrope electrolytes increase the solubility of redox-active materials. It was confirmed that the mechanism of hydrotrope action varies depending on the structure of the active material, and guidelines for molecular structures optimized for each mechanism were presented. The intermolecular nuclear Overhauser effect is a method that measures the distance between hydrogen atoms within molecules by utilizing nuclear spin transfer between hydrogen atoms present in different molecules.
Professor Yoo said, “We have been able to improve the chronic limitation of low solubility of redox-active organic molecules, and we expect this to contribute not only to the development of high-capacity, high-output redox batteries but also to the development of various molecular-structured energy storage materials. In the future, leveraging the advantages of using liquids as energy storage media will also aid in the development of applied technologies such as large-scale ESS.”
The research results were published on the 21st of last month in ACS Energy Letters, a prestigious international journal in the fields of organic chemistry and energy.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.















