Irregular Heart Rhythm Function
Obtains US FDA Approval
The new Galaxy Watch series from Samsung Electronics, set to be released in the second half of the year, will feature an 'Irregular Heart Rhythm Notification' function.
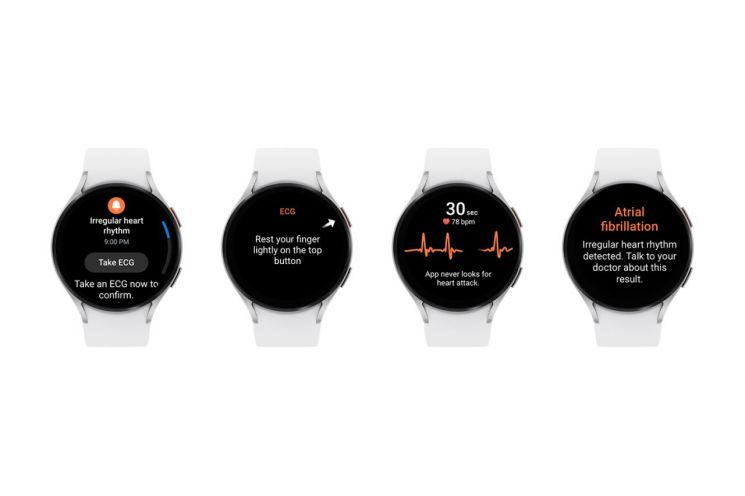
Samsung Electronics announced on the 9th that the 'Irregular Heart Rhythm Notification' feature of the Samsung Health Monitor application has received approval from the U.S. Food and Drug Administration (FDA). Irregular heart rhythms are detected through the Galaxy Watch's 'BioActive Sensor.' When a Galaxy Watch that supports this feature continuously detects the user's irregular heartbeat, it interprets this as an abnormal sign and notifies the user via the watch display that there is a possibility of 'atrial fibrillation.'
It additionally prompts the user to activate the 'Electrocardiogram (ECG)' function embedded in the Galaxy Watch for accurate heart rate measurement. Users can also check the history of irregular heart rhythms by accessing the 'ECG Monitoring' feature in the Samsung Health Monitor app.
Hong Pak, Head of the Digital Health Team at Samsung Electronics' MX Division (Executive Director), said, "The irregular heart rhythm notification feature, which has obtained U.S. FDA approval, can help people who are unaware of heart-related risk situations," adding, "Samsung Electronics will continue to strive to develop various monitoring technologies that can protect customers' health."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.