Phase 3 Clinical Trial Results Released
Rebound After FDA Accelerated Approval Denial in January
"FDA Approval Application to Be Submitted Within First Half of the Year"
Donanemab, an Alzheimer's disease treatment developed by Eli Lilly, has shown successful results in Phase 3 clinical trials, signaling a green light for approval by the U.S. Food and Drug Administration (FDA).
On the 3rd (local time), Lilly announced that donanemab, in the Phase 3 clinical trial (TRAILBLAZER-ALZ 2) targeting early Alzheimer's patients, delayed the decline in cognitive and daily living abilities by 35% compared to the placebo group and reduced the risk of progression to the next stage of the disease by 39%.
This clinical trial involved 1,182 patients with early Alzheimer's disease, including those with mild cognitive impairment (MCI). The primary efficacy endpoint was the integrated Alzheimer's Disease Rating Scale (iADRS), which evaluates cognitive function and daily living abilities such as driving, hobbies, and asset management. The treatment group showed a 35% reduction in decline in these abilities. The Clinical Dementia Rating-Sum of Boxes (CDR-SB), a commonly used secondary endpoint to assess cognitive function, also showed a similar 36% reduction in decline over 18 months.
Given donanemab’s mechanism of targeting symptom improvement through the removal of accumulated Aβ plaques, the most concerning side effect, amyloid-related imaging abnormalities (ARIA), appeared similarly to previous Phase 2 trial results. 'ARIA-E,' such as brain edema, occurred in 24.0% of the treatment group. 'ARIA-H,' including cerebral microbleeds, was observed in 31.4% of the treatment group, higher than the 13.6% in the placebo group. Severe ARIA occurred in 1.6% of patients. Among participants, two died due to ARIA, and one died after experiencing severe ARIA.
Daniel Skovronsky, Lilly’s Chief Scientific and Medical Officer, stated, "This trial is the first Phase 3 study to delay cognitive and functional decline in Alzheimer's disease by 35%. We are very pleased that donanemab has demonstrated statistically significant positive clinical results for patients with Alzheimer's disease."
Based on these results, Lilly plans to request FDA approval within the first half of the year and apply for marketing authorization from other regulatory agencies as soon as possible. If officially approved by the FDA, donanemab will become the third antibody treatment for Alzheimer's disease following Biogen and Eisai’s Aduhelm (active ingredient aducanumab) and Leqembi (active ingredient lecanemab). Detailed full clinical results will be disclosed at the Alzheimer's Association International Conference (AAIC) and in academic journals in July.
'Brilliant Revival' Just Four Months After Accelerated Approval Rejection
Although Lilly’s accelerated FDA approval was rejected in January, the company overcame this setback and achieved successful Phase 3 clinical results, marking a comeback. The FDA denied approval after reviewing results up to Phase 2, citing a limited number of patients with long-term follow-up of more than 12 months.
However, Lilly explained that the lack of data was due to donanemab’s strong efficacy. Because the clinical design required stopping the trial once the Aβ plaque removal reached a predetermined level, treatment was discontinued quickly, preventing long-term follow-up.
In fact, according to the results released by Lilly, about half (47%) of the donanemab treatment group showed no decline in CDR-SB during the first year of treatment, indicating no disease progression, compared to only 29% in the placebo group.
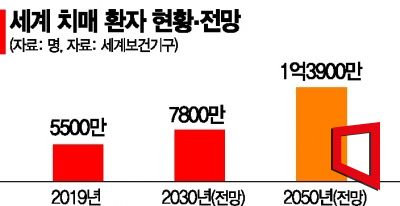
With the rapid increase in dementia patients, the market outlook for donanemab after its launch is also expected to be bright. Pharmaceutical research firm Kotelis projected that the Alzheimer's treatment market will grow from $1.6 billion (approximately 2 trillion KRW) in 2020 to $5.7 billion in 2030 and $20 billion (approximately 27 trillion KRW) in 2050, with an average annual growth rate of 29%. As antibody treatments continue to be developed, the current prescription share of acetylcholinesterase inhibitors, which accounts for 47%, is expected to be dominated by Aβ antibody treatments such as donanemab and Leqembi, reaching 69% by 2025.
Global academic information service company Clarivate also listed donanemab among the top 15 drugs expected to become blockbuster medicines with annual sales exceeding $1 billion by 2027 among new drugs launching or likely to be approved this year. Donanemab is projected to generate $1.34 billion (approximately 1.789 trillion KRW) in sales, ranking 4th. Leqembi is also expected to achieve $1.02 billion (approximately 1.361 trillion KRW) in sales, ranking 8th.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.