Institute for Basic Science Develops Technology to Recover All Metals with a Single Reactor
Significant Savings in Time and Cost, Enabling Economic Feasibility
Recycling spent batteries can simultaneously achieve cost savings, resource acquisition, and environmental preservation. This technology is especially crucial for resource-poor countries like South Korea, which lack battery materials such as lithium, nickel, and cobalt. Domestic researchers have developed a spent battery metal recovery technology that can recover all metals with a single reactor, significantly saving time and costs.
The Institute for Basic Science (IBS) announced on the 3rd that a research team led by Bartosz Grzybowski, group leader of the Center for Soft and Living Matter (and distinguished professor at UNIST), developed a new method to recycle metals from spent lithium-ion batteries using a single reaction vessel. This method can simplify the complex recycling process, greatly reducing time and costs.
Lithium-ion batteries are a type of secondary battery capable of charging and discharging. They are made from lithium, the lightest metal element, making them lightweight with high energy density. Widely used in mobile phones, laptops, electric vehicles, and more, their demand is expected to increase further. However, the growing volume of lithium-ion battery waste poses a disposal challenge. Batteries that have reached the end of their life due to repeated charging and discharging must be replaced, but the leftover spent batteries cannot simply be discarded in landfills or incinerators. There is a high risk of explosion and fire, and toxic substances can leak out, contaminating soil and water, causing environmental and safety issues.
Therefore, there is growing interest in technologies that extract and recycle valuable raw materials such as lithium, nickel, and cobalt from spent batteries. However, existing recycling processes are complex and costly, which is one reason for the currently low recycling rate. Most spent batteries are finely shredded and crushed into a black powder, then undergo chemical treatment to filter out the metal raw materials. First, the black powder is transferred to a cylindrical tank for chemical processing, where an extractant that binds with acid or specific metals is added and mixed. Just as water and oil separate into layers, the extractant containing dissolved specific metals separates from the solution containing the other metals. Then, only the extractant containing the specific metals is removed to extract the metals. This sequence is repeated to separate metals using extractants for each raw material. Because the existing process requires multiple reaction vessels and different reaction conditions, it is complicated. Many studies have attempted to separate and extract metals in a single cylinder using membranes, but there were limitations such as membrane rupture during vigorous mixing.
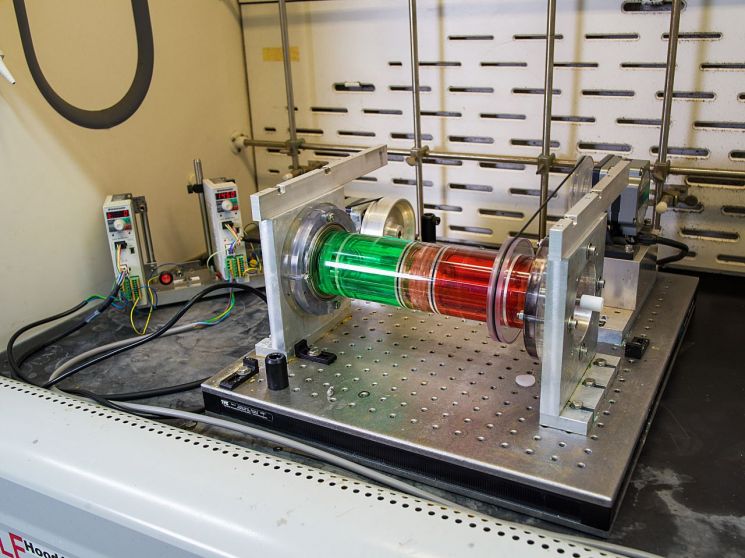
The research team improved efficiency by applying a previously developed chemical process technology to spent battery recycling. In 2020, the team developed a chemical synthesis system capable of easily handling multiple chemical processes in a single reaction vessel. Based on the fact that solutions with different densities do not mix but stack in layers, they placed several solvents inside a rotatable cylinder and used these solvents to move or separate reactants, significantly reducing the time required for conventional chemical synthesis processes.
The newly developed rotating reactor can separate and extract metal mixtures at once without passing through multiple vessels in a horizontal form. Inside the vessel, there are three layers: a feed layer supplying the metal mixture, an acceptor layer receiving the separated metals, and a shuttle layer preventing mixing between the two. The feed layer is highly acidic, while the acceptor layer has low acidity. The shuttle layer contains an organic solvent that selectively separates metals by reciprocating between the feed and acceptor layers.
When a metal mixture is added to the feed layer and strong rotation is applied, each layer maintains its form without collapsing, similar to how water and oil do not mix. Due to the extractant in the shuttle layer, lithium and nickel remain in the feed layer, while manganese and cobalt move to the acceptor layer. Unlike single reactors using membranes, this reactor has the advantage of withstanding vigorous mixing without any issues.
First author Senior Researcher Cristobal Quintana said, “Compared to existing metal separation and extraction methods, this technology can quickly filter desired metals using much lower concentrations of metal extractants.” He added, “This technology has very high utility as it can be suitably applied to separate various metals beyond those used in batteries.”
The research results were published online on March 16 in the international journal Advanced Materials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.