Daewoong Achieves Technology Export Success for Autoimmune Disease New Drug
Advance Payment of 14.8 Billion KRW... Up to 640 Billion KRW Scale
Kakao Healthcare and Welt Sign MOU
Kakao Healthcare Accelerates Blood Glucose Management Business Using CGM
President Yoon Suk-yeol's state visit to the United States appears to have further accelerated the global expansion of domestic pharmaceutical, bio, and healthcare companies. Not only were export contracts for new drug candidate substances signed, but various agreements were also established to serve as footholds for entry into the U.S. market in the digital healthcare sector.
 Daewoong Pharmaceutical has signed a technology export contract for autoimmune disease treatments with Vitali Bio, a subsidiary of Editum Bio.
Daewoong Pharmaceutical has signed a technology export contract for autoimmune disease treatments with Vitali Bio, a subsidiary of Editum Bio. [Photo by Ministry of Health and Welfare]
At the 'Korea-U.S. Digital & Biohealth Business Forum' held on the 27th (local time) in Boston, USA, hosted by the Ministry of Health and Welfare and the Korea Health Industry Development Institute, one export contract for a new drug candidate substance and nine memorandums of understanding (MOUs) for cooperation were signed. According to the government, this is expected to generate an economic effect exceeding 670 billion KRW, with even greater long-term economic benefits anticipated.
The main player in the technology export is Daewoong Pharmaceutical. It signed a global technology export contract for the oral treatment 'DWP213388' for autoimmune diseases with Vitali Bio, a subsidiary of Additum Bio. This substance is the first-in-class drug developed by Daewoong Pharmaceutical, which received approval for its clinical phase 1 trial plan (IND) from the U.S. Food and Drug Administration (FDA) in August last year. Unlike existing treatments that inhibit either B cells or T cells, this 'dual-target inhibitor' simultaneously inhibits both cell types to enhance efficacy. It has been confirmed in animal models to have superior therapeutic effects compared to existing drugs for autoimmune diseases such as rheumatoid arthritis.
The contract is valued at up to $477 million (approximately 641.1 billion KRW), including an upfront payment of $11 million (about 14.8 billion KRW). Additionally, royalties based on net sales will be paid. Jeon Seung-ho, CEO of Daewoong Pharmaceutical, who attended the signing ceremony, stated, "We are strengthening our research and development (R&D) investments and actively pursuing an open innovation strategy including technology exports to become a global innovative new drug development company based in the U.S. Based on this contract, we will enhance cooperation with advanced companies in the U.S. and develop a diverse product portfolio to the global commercialization stage."
Kakao Healthcare, Promoting 'Blood Sugar Management' and 'Data Enabler'... Establishing a Foundation for Actual Business
In the digital healthcare sector, Kakao Healthcare signed three MOUs, and Welt, a company developing digital therapeutics (DTx), signed one MOU.
Kakao Healthcare, which proposed mobile-based patient blood sugar management service 'Gamma' and 'Delta,' a data sharing and utilization tool without data leakage, as its main business models, laid the groundwork for its business by having CEO Hwang Hee personally attend the site to sign the MOUs.
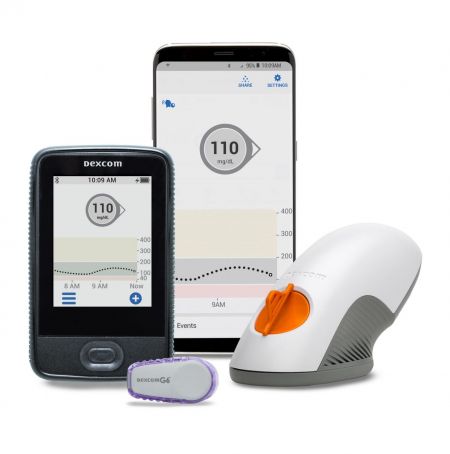
For Gamma, an MOU was signed with Dexcom, a global leader in continuous glucose monitoring (CGM), to provide innovative mobile services and support global expansion. They plan to collaborate on software development that utilizes data derived from Dexcom's CGM to offer blood sugar and health management services. Dexcom is a leading global CGM company ranked number one in the U.S. market. Following the G6, its latest model G7 received FDA approval in December last year and is sold in the U.S., the U.K., and other countries. In Korea, Huons handles sales, maintaining over 70% market share in the CGM market for type 1 diabetes patients.
Unlike traditional blood sugar measurement methods that require cumbersome processes such as finger pricking, CGM is attached to the arm or abdomen and continuously measures blood sugar automatically, greatly enhancing patient convenience. One application can continuously and in real-time measure blood sugar for about 10 to 15 days without the need for blood sampling.
This is not Kakao Healthcare's first CGM-related MOU. Last month, it also signed an MOU with i-SENS, a domestic CGM developer, for chronic disease management using CGM. Kakao Healthcare plans to launch CGM-related services in the third quarter.
For the Delta project, an MOU for technical cooperation was signed with Google Cloud. The plan is to develop a healthcare data analysis and prediction system using federated learning technology, creating a platform that enables AI research on medical data for hospitals, research institutions, and companies without the risk of data leakage. CEO Hwang Hee's vision of becoming a 'data enabler'?helping to use data well without collecting it?is becoming more concrete. Additionally, Kakao Healthcare signed a 'term sheet' with Signature Healthcare, which operates 19 mental health institutions across the U.S., for initial validation of its remote patient monitoring solution. After validation, the plan is to expand to Signature Healthcare-operated hospitals and enter the U.S. remote patient monitoring market through joint ventures.
Welt, whose CEO Kang Sung-ji participated as an official member of the economic delegation, signed an MOU with Northeastern University in the U.S. for industry-academia cooperation and technology transfer in healthcare, global education linkage, and local recruitment of outstanding talent. Northeastern University, located in Boston, is recognized for its strengths in digital healthcare, including offering a digital healthcare innovation degree program in collaboration with the Mayo Clinic. The MOU also includes discussions on establishing a Northeastern University industry-academia cooperation center in Korea.
In addition, in the pharmaceutical and bio sectors, UbioLogics agreed to export the conjugate protein key raw material 'CRM197' for pneumococcal vaccine development to Aeolian Biotech, and OliX signed an MOU with DynamiCure Biotechnology for joint research and development of gene therapies based on RNA interference (RNAi) technology. In medical devices, PCL promised to strengthen technical cooperation on in vitro diagnostics (IVD) products and collaborate on U.S. market entry with ARC Group, while GMS Healthcare signed an MOU to enter the U.S. public procurement market for medical refrigeration and freezing products through local procurement company Westcarb.
Furthermore, in the industry-academia cooperation sector, Yonsei University, which operates the Korean version of NIBRT (K-NIBRT), signed an MOU with the Korea Health Industry Development Institute and Moderna for education and exchange cooperation to train personnel in bio-processes, messenger RNA (mRNA) vaccines, biotechnology, and related fields. The plan is to strengthen K-NIBRT's bio-process personnel training programs based on Moderna's expertise in the mRNA field.
Boston, the 'World's Largest Bio Cluster'... Will the C&D Office Become a Launchpad for Korean Companies?
Meanwhile, the forum held to strengthen cooperation between Korean and U.S. companies in the digital and biohealth sectors on the occasion of President Yoon Suk-yeol's state visit was attended by 100 people, including Lim In-taek, Director of the Health and Medical Policy Office at the Ministry of Health and Welfare; Cha Soon-do, President of the Korea Health Industry Development Institute; Korean companies residing in the C&D Incubation Office at the Cambridge Innovation Center (CIC) in Boston; Korean and U.S. companies and institutions signing contracts and MOUs; local companies; and experts. Among the 122 members of the U.S. economic delegation, 21 (17.2%) were from bio and digital healthcare companies and institutions, representing a significant proportion.
Before the signing ceremony, case presentations were also held. Ko Jong-sung, CEO of Genosco, who achieved success such as developing the globally blockbuster drug 'Reclaza' (active ingredient: Lazertinib) and licensing it to Yuhan Corporation, presented a case study on how he utilized the Boston bio-ecosystem after becoming the first Korean to enter Boston in 2008.
Following this, resident companies of the C&D Incubation Office such as Yuhan USA, Huons USA, and Dong-A ST presented and shared their U.S. market entry strategies and achievements since moving in. The C&D Incubation Office, which opened in June last year, currently houses 20 companies supported by the government, including Voronoi, Aribio, Welt, Yuhan USA, Ildong Pharmaceutical, Generos, Huons USA, JW Ceriac (JW Pharmaceutical's U.S. research corporation), Dong-A ST, Standigm, Organoid Science, HanAll Biopharma, Medisapiens, Medipixel, Biotoxtech, Avion, U.S. Bio Global, Intec, Genuv, and Hi.
Lim In-taek said, "It was meaningful to hold a forum where companies leading new market development through cooperation with global companies gathered in Boston, a symbolic place for the digital and biohealth sectors. We will faithfully implement the 'Biohealth New Market Creation Strategy' announced last February and continue efforts to support the successful U.S. entry of our companies." Cha Soon-do added, "The C&D Incubation Office serves as an advanced base for Korean digital and biohealth companies entering the U.S. and is becoming a place for creating results through innovation. We will actively support continued cooperation outcomes."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.