Professor Tae-eun Park's team from the Department of Biomedical Engineering at UNIST has developed a microphysiological system that mimics the physiological and pathological characteristics of human white adipose tissue.
Adipose tissue is a type of connective tissue in the body mostly composed of adipocytes. Among them, white adipose tissue plays a crucial role in storing energy and maintaining homeostasis in the body, and functions as an endocrine organ by secreting various substances such as fatty acids and hormones.
Obesity, caused by excessive fat accumulation in white adipose tissue, is the leading cause of death worldwide. Moreover, obesity can lead to various complications such as diabetes and cardiovascular diseases, and is also associated with the occurrence and progression of certain cancers.
As global interest in obesity grows, models that can replicate the physiological and pathological characteristics of adipose tissue are being developed. However, existing culture methods have faced difficulties in differentiating adipocytes containing fat lumps in the form of single droplets and maintaining the functionality of differentiated cells.
Furthermore, there have been limitations in mimicking the microenvironment of adipose tissue and reproducing its physiological and pathological features.
 Schematic diagram of an obesity AT MPS model that established a culture platform for adipocytes using decellularized adipose tissue.
Schematic diagram of an obesity AT MPS model that established a culture platform for adipocytes using decellularized adipose tissue.
Under these conditions, the research team successfully developed an adipose tissue microphysiological system that mimics the physiological and pathological characteristics of obese adipose tissue.
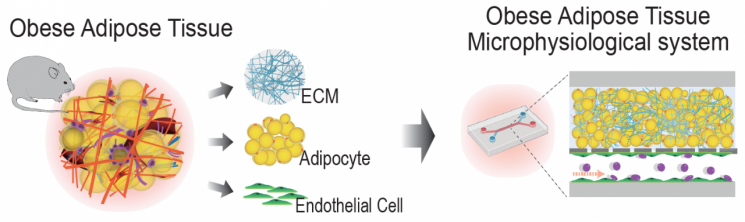
The developed microphysiological system consists of an upper microchannel where adipocytes are cultured in three dimensions within an extracellular matrix (ECM)-based hydrogel structure isolated from adipose tissue, and a lower microchannel co-cultured with adipose endothelial cells.
The extracellular matrix is a three-dimensional structure that fills the space between various cells in tissues and is composed of molecules secreted and accumulated by cells. In particular, the extracellular matrix of adipose tissue dynamically changes its composition and characteristics during the obesity process, directly and indirectly regulating adipocyte behavior.
First, the research team used a decellularization technique on normal and obese adipose tissues to create hydrogels composed of extracellular matrix.
The formed hydrogels retained the tissue-specific physiological and pathological characteristics, accurately mimicking the unique microenvironments of normal and obese adipose tissues. They also helped maintain the functionality of cultured primary adipocytes over a long period.
The team fabricated microphysiological systems for both obese and normal adipose tissues and reproduced the dysfunction observed in vascular endothelial cells within obese adipose tissue.
Inflammatory responses increased by obesity activate vascular endothelial cells and increase the number of immune cells within adipose tissue. These pathological features were observed in the developed obese adipose tissue microphysiological system as approximately twice the immune cell adhesion.
The research team also investigated the unclear association between obesity and cancer.
Using the developed adipose tissue microphysiological system, they confirmed that under obese conditions, cancer cell mobility and adhesion to tissue increased by about twofold. This demonstrated that direct interactions between obese adipose tissue and cancer cells can be easily visualized and quantified.
 Researcher Heejeong Yoon (left) and Professor Taeun Park, who conducted this study, are taking a photo.
Researcher Heejeong Yoon (left) and Professor Taeun Park, who conducted this study, are taking a photo.
Hee-jung Yoon, the first author of the study, said, “This research mimicked the microenvironment of obese adipose tissue. By using the microphysiological system, it is possible to observe the activation, inflammation, dysfunction of vascular endothelial cells caused by obese tissue, and their interactions with other cells, which can be used to elucidate various disease mechanisms related to fat or to develop obesity treatments.”
This study was published on January 29, 2023, in the international journal in the field of biomaterials, Acta Biomaterialia.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















