Speeding Up Strategies Including Drug Technology Exports
Largest Scale in Latin America... Increasing Demand Trend
[Asia Economy Reporter Myunghwan Lee] The domestic pharmaceutical and bio industry is accelerating its efforts to penetrate the Brazilian market worth 27 trillion won.
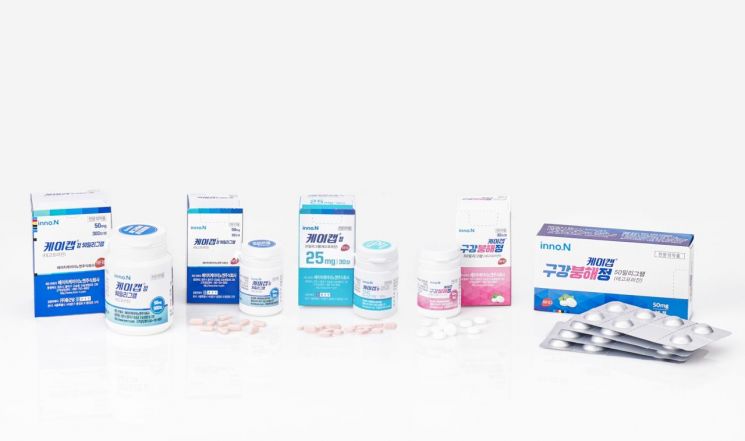
On the 26th, HK Innoen announced that it had signed a technology export contract for the gastroesophageal reflux disease new drug ‘K-CAB’ (active ingredient: Tegoprazan) in Brazil. Under this contract, HK Innoen will transfer the manufacturing technology of K-CAB to Eurofarma, the third-largest pharmaceutical company locally. The contract is known to include technology fees at the approval and launch stages as well as royalties based on sales. The market size for peptic ulcer drugs in Brazil was estimated at about 800 billion won annually as of 2020, ranking sixth in the world.
Celltrion Healthcare also recently succeeded for the second consecutive year in winning the Brazilian government’s pharmaceutical supply project for the autoimmune disease treatment drug ‘Remsima’ (active ingredient: Infliximab) in Brazil. The supply volume is 342,000 vials, and considering general supply quantities, Celltrion Healthcare explained that its local market share is expected to exceed 80%.
Earlier, in August last year, L&K Bio signed a spinal implant supply contract worth 40 million dollars (about 49 billion won) with a local distributor in Brazil. In July of the same year, SK Biopharm signed a technology export contract worth 81 billion won for the epilepsy new drug ‘Cenobamate’ with a Brazilian pharmaceutical company. Daewoong Pharmaceutical’s botulinum toxin product ‘Nabota’ is also reported to have grown more than threefold in sales in Brazil compared to the previous year.
According to the Korea Health Industry Development Institute, the Brazilian pharmaceutical market is about 22.5 billion dollars (about 27 trillion won), making it the largest in Latin America. Globally, it ranks around eighth, accounting for about 3% of the world market. The steady increase in demand for biopharmaceuticals locally is also seen as an opportunity factor. This is supported by the Brazilian government’s expansion of public healthcare coverage, as well as the increasing elderly population and the rising incidence of chronic diseases. According to KOTRA’s S?o Paulo Trade Center, sales in the Brazilian pharmaceutical market increased by about 26% compared to the previous year. The fact that Brazil’s pharmaceutical market imports amount to ten times its exports, showing heavy reliance on imports, also enhances the attractiveness of market entry. On the 17th, the Korea Pharmaceutical and Bio-Pharma Manufacturers Association held a briefing session on entering the Brazilian pharmaceutical market jointly with the Embassy of Brazil in Korea and KOTRA’s S?o Paulo Trade Center. The briefing, conducted for domestic bio companies and related organizations, covered opportunity factors for entering Brazil and regulatory approval procedures.
However, it should be noted that the local certification process is complicated and time-consuming. Certification by the National Health Surveillance Agency (ANVISA), which plays a role similar to Korea’s Ministry of Food and Drug Safety, is essential, and local business registration is mandatory for certification. The certification process is complicated and lengthy, and the requirement to renew certification periodically can also be a burden.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.