"Over 30% Performance Improvement Compared to Existing Lithium-Ion Batteries"
[Asia Economy Reporter Kim Bong-su] Domestic researchers have succeeded in developing a next-generation 'lithium-sulfur battery' with performance more than 30% better than existing batteries.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 19th that Professor Jinwoo Lee's research team from the Department of Bio and Chemical Engineering, in collaboration with Professor Jungwoo Han's team at Pohang University of Science and Technology (POSTECH) and LG Energy Solution's Next-Generation Battery Research Center, succeeded in developing a lithium-sulfur battery with significantly improved energy density and lifespan stability compared to existing models.
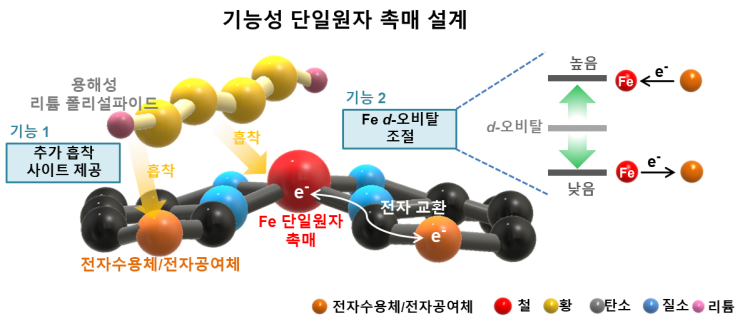 Schematic diagram of the lithium-sulfur battery developed by KAIST-LG Energy Solution, illustrating the strategy to induce electron exchange phenomena by introducing electron donors and acceptors around iron atoms. Image courtesy of KAIST.
Schematic diagram of the lithium-sulfur battery developed by KAIST-LG Energy Solution, illustrating the strategy to induce electron exchange phenomena by introducing electron donors and acceptors around iron atoms. Image courtesy of KAIST.
Lithium-sulfur batteries are expected to achieve energy densities 2 to 3 times higher than commercial lithium-ion batteries, attracting considerable attention as candidates for next-generation secondary batteries. Especially in applications where the amount of energy stored at once is critical, such as electric vehicles and electronic devices, the importance of lithium-sulfur battery technology development is increasingly emphasized.
To realize lithium-sulfur batteries with high energy density, it is essential to reduce the amount of heavy electrolyte inside the battery while securing high capacity and operating voltage. However, when the amount of electrolyte inside the battery decreases, the contamination of the electrolyte caused by the dissolution of lithium polysulfides at the cathode becomes severe, lowering lithium-ion conductivity and reducing electrochemical conversion reaction activity, which limits the achievement of high capacity and operating voltage.
Worldwide, many researchers have developed various functional materials to improve the continuous dissolution of lithium polysulfides and the activity of conversion reactions, but so far, it has been difficult to secure high energy density and lifespan stability at the lithium-sulfur pouch cell level. A pouch cell is a battery form where materials such as cathode, anode, and separator are stacked and then packaged in a film. Pouch cells are considered one of the most advanced types of batteries and have the advantage of being manufacturable in various shapes depending on the application.
The research team succeeded in developing an iron (Fe) atom-based functional cathode material that can significantly improve the dissolution phenomenon of lithium polysulfides and electrochemical conversion reactivity. By introducing an optimized electronic structure iron atom-based functional material into the cathode, the team was able not only to efficiently suppress the dissolution of lithium polysulfides but also to improve the reactivity that allows lithium polysulfides to convert into insoluble lithium sulfides. This enabled the realization of high reversible capacity, operating voltage, and lifespan stability even with a small amount of electrolyte inside the battery.
In particular, they achieved an energy density of over 320 Wh kg-1 (energy stored per unit weight) in a lithium-sulfur pouch cell with an A h level energy density about 30% higher than that of existing commercial lithium-ion batteries. Since iron (Fe) is a very inexpensive material, the cathode functional material developed in this study also holds potential for future use in the lithium-sulfur battery industry.
Professor Jinwoo Lee stated, "In developing excellent lithium-sulfur battery cathode functional materials, we demonstrated that electronic structure control technology induced by electron exchange phenomena can be very promising," adding, "Efforts to secure high energy density and lifespan stability at the lithium-sulfur pouch cell level should continue through the development of various technologies that can control the electronic structure of functional materials."
The results of this study were published online on December 17 last year in the international academic journal 'Advanced Materials.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















