[Asia Economy Reporter Lee Chun-hee] Last year, attempts to develop domestic software medical devices (SaMD) increased, and the target diseases also diversified.
The Ministry of Food and Drug Safety disclosed on the 13th the status of clinical trial plan approvals for SaMD, including digital therapeutic (adjunct) devices (DTx), and the status of approved products by field last year. SaMD refers to medical devices developed and manufactured solely with software that have been authorized, certified, or reported. They are broadly categorized into ▲‘diagnostic assistance and prediction’ of diseases ▲‘treatment and alleviation’ (DTx) of diseases and symptoms ▲‘surgical treatment and assistance’ fields.
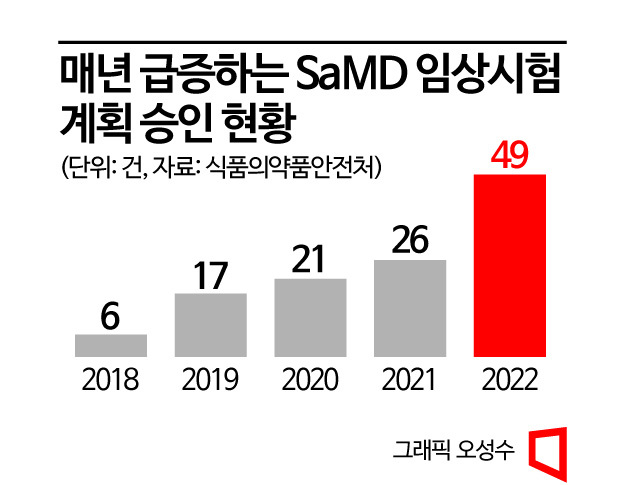
The number of approved clinical trial plans for SaMD has surged annually in recent years. Over the past five years from 2018 to last year, it increased more than eightfold from 6 to 49 cases. In particular, last year saw 49 cases, about twice the 26 cases of the previous year. By field, AI-based software for diagnostic assistance and prediction accounted for the largest number with 31 cases, followed by 17 cases in DTx, and 1 case in surgical treatment and assistance, including augmented reality (AR)-based surgical assistance software.
In diagnostic assistance and prediction, approvals were granted for products using AI to assist diagnosis of acute cerebral infarction occurrence and location using brain computed tomography (CT) images, assist detection of pancreatic cancer areas in ultrasound images, and predict the risk of myocardial infarction within one year by analyzing medical records of cardiovascular disease patients.
In the DTx field, while 9 clinical trials were approved in 2021 for 8 diseases, last year showed quantitative and qualitative growth with 17 clinical trials approved for 12 diseases. Approved products included those that improve attention deficit in pediatric patients diagnosed with attention deficit hyperactivity disorder (ADHD) based on game platforms, and products that analyze cognitive status of mild cognitive impairment (MCI) and provide cognitive therapy to improve cognitive function. A Ministry of Food and Drug Safety official explained, "Until now, DTx development mainly focused on products for insomnia or addiction symptom alleviation. Compared to this, last year it was confirmed that applications for a wider variety of diseases such as ADHD, MCI, and developmental disorders were attempted."
By clinical trial phase, there were 16 exploratory clinical trials in the early phase and 33 confirmatory clinical trials in the late phase. Unlike drug clinical trials, which are usually divided into phases 1 to 3, medical device clinical trials are conducted in two stages: exploratory and confirmatory. Exploratory trials are conducted over a relatively short period with a small number of subjects, while confirmatory trials involve a larger number of subjects to secure statistical significance. However, the Ministry of Food and Drug Safety stated, "Among these, 9 trials have been completed, 18 are ongoing, and 22 have not yet started," adding, "It is expected that more time will be needed before these products are actually used in medical practice."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.