Institute for Basic Science - University of Science and Technology of China Joint Research Team
[Asia Economy Reporter Kim Bong-su] Domestic scientists have developed a new carbon material that behaves as a semiconductor at room temperature and exhibits metallic properties at low temperatures.
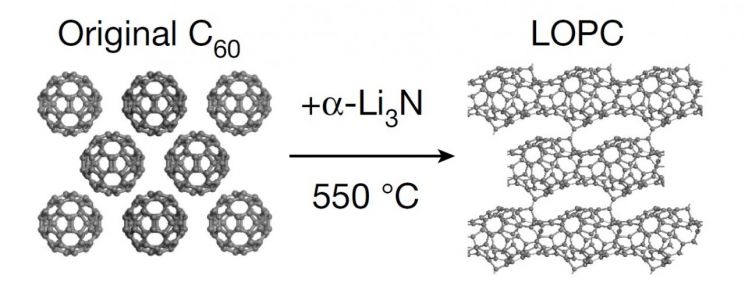
The Institute for Basic Science (IBS) announced on the 12th that the research team led by Rodney Ruoff, head of the Multidimensional Carbon Materials Research Division and distinguished professor at Ulsan National Institute of Science and Technology, in collaboration with the University of Science and Technology of China, synthesized a new carbon material. The team named this material ‘LOPC (Long-range Ordered Porous Carbon)’ due to its carbon atoms being arranged in a regular porous structure over a large area.
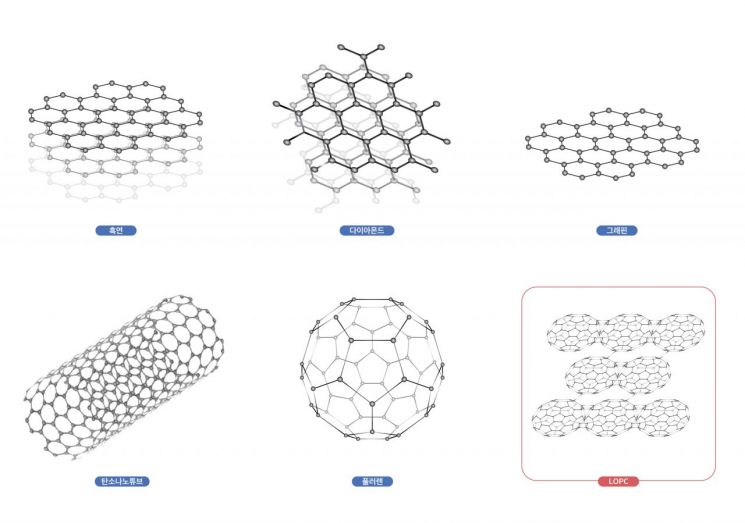
The discovery of this new carbon structure holds high academic and industrial value. Carbon materials such as pencil lead, diamond, and graphene exhibit diverse physical properties depending on the arrangement of atoms. They also have a close connection to the Nobel Prize. The 1996 Nobel Prize in Chemistry was awarded for the development of ‘fullerene,’ a structure composed of 60 carbon atoms forming a cage, and the 2010 Nobel Prize in Chemistry recognized the development of graphene.
Unlike graphene, fullerene has not been extensively studied for applications. To apply fullerene, its atomic arrangement or structure must be modified. However, with a diameter of only 0.7 nm (nanometers; 1 nm is one billionth of a meter) and a stable molecular structure, chemical and physical modifications are difficult. Even if modification succeeds, there remains the challenge of synthesizing it in large quantities at a level valuable for applications.
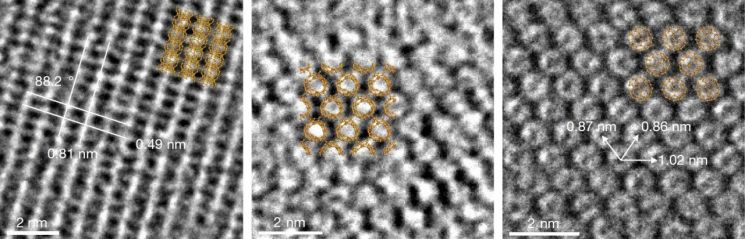
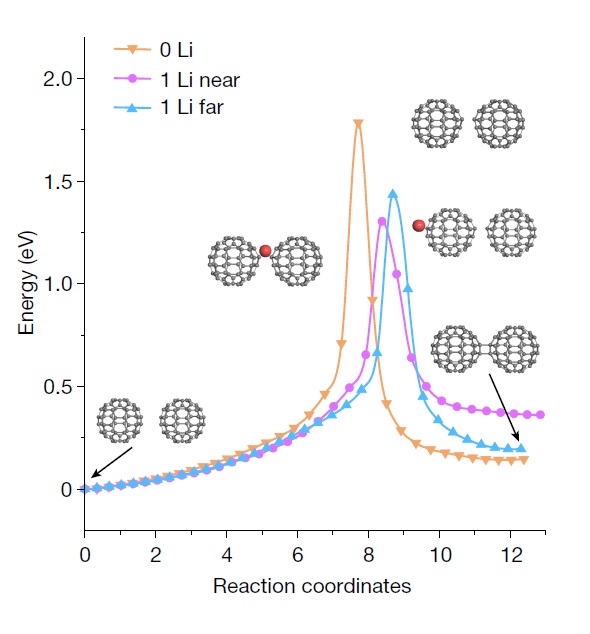
The research team synthesized a new carbon material using fullerene. When powdered fullerene was mixed with alpha-lithium nitride compound (α-Li3N) and heated up to 550°C, some bonds between carbon atoms in the fullerene broke, and adjacent fullerenes bonded and connected. This can be easily imagined as cutting a soccer ball with scissors and joining several pieces together. Using advanced analytical equipment to analyze the synthesized structure, it was confirmed that LOPC is a structure formed during the transformation of the three-dimensional fullerene into a two-dimensional material like graphene.
The team then analyzed the physical properties of LOPC. Despite using fullerene, which has low electrical conductivity, LOPC exhibited electrical conductivity at the level of semiconductor devices at room temperature. At temperatures below 30K (-243.15°C), it showed metallic-level electrical conductivity.
Professor Yanwu Chu of the University of Science and Technology of China said, “German mathematician Hermann Schwarz proposed the possibility of structures with negative curvature while studying soap bubble surfaces, and many scientists have tried to synthesize carbon materials with this structure, called ‘carbon Schwarzite.’ Inspired by the prior research of Ruoff’s team, we were able to synthesize a new material resembling the bonding of carbon Schwarzite.”
Until now, developed carbon materials have been either planar like graphene or convex like fullerene. The possibility of synthesizing concave carbon materials with negative curvature, like a horse saddle, was proposed in the 1990s. However, even after more than 30 years, such materials have not been synthesized.
Generally, carbon atoms chemically bond with four surrounding atoms (tetravalent bonding), but to realize negative curvature, a ‘trivalent bonding’ structure is required, where carbon atoms bond with three other atoms. In 2010, Ruoff reported the first synthesis of trivalent bonded carbon in the international journal Advanced Materials. Based on this, Chinese researchers succeeded in synthesizing LOPC with trivalent bonding in this study.
The synthesis of LOPC is regarded as an important clue for synthesizing carbon Schwarzite. Carbon Schwarzite is expected to have significant industrial applications such as capacitors that can store large amounts of energy, carriers that deliver drugs inside the body using internal void spaces, and highly efficient catalysts with large surface areas.
Ruoff said, “This study is the first case of synthesizing the new material LOPC in large quantities at the gram (g) scale and clearly identifying its structure, and it can be expanded to the kilogram (kg) scale in the future. We have taken a step closer to synthesizing the ideal material, ‘carbon Schwarzite.’”
The research results were published online in the international journal Nature (IF 69.504) on the same day.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















