Joint Research Result in Mathematics and Pharmacy in Korea
Existing Formula for Drug Interaction Verification Found to Have Errors
New Formula Proposed with Twice the Accuracy
 Photo by IBS
Photo by IBS
[Asia Economy Reporter Kim Bong-su] Domestic researchers have revealed that the drug interaction prediction formula used by the U.S. Food and Drug Administration (FDA) is incorrect.
The Institute for Basic Science (IBS) announced on the 4th that the CI research team of the Mathematical and Computational Science Research Division led by Kim Jae-kyung, in collaboration with Professor Chae Jung-woo and Professor Kim Sang-gyeom from the College of Pharmacy at Chungnam National University, identified the cause of the inaccuracy in the FDA's drug interaction prediction formula and proposed a new formula that improves accuracy by more than twice.
Drugs absorbed into the body are metabolized by enzymes in various organs, including the liver, and eliminated from the body. When two or more drugs are taken together, one drug can alter the metabolism of another, either accelerating or inhibiting its excretion. This can result in the failure to achieve the intended therapeutic effect or cause side effects. This phenomenon is called "drug-drug interaction (DDI)."
Accurately predicting the rate of drug elimination based on drug interactions is crucial for drug prescription and new drug development. Medical professionals prescribe medications based on drug interaction information specified in drug labels when administering combination therapies. In the new drug development process, studying and indicating drug interactions is mandatory.
The FDA first issued guidance to evaluate drug interactions and minimize side effects during polypharmacy in 1997 (revised in January 2020). Since it is practically impossible to evaluate interactions between new drug candidates and all marketed drugs during development, the FDA recommends using the formula presented in the guidance to indirectly assess drug interactions.
The problem is that this formula lacks accuracy. The FDA's formula is based on the Michaelis-Menten equation, which describes enzyme reaction rates. This formula assumes that the concentration of enzymes involved in drug metabolism in the body is low. The research team found that the actual enzyme concentration involved in drug metabolism in the liver is more than 1,000 times higher than the value used for predictions, identifying the cause of the FDA formula's inaccuracy.
Professor Chae Jung-woo of Chungnam National University College of Pharmacy said, "Researchers have been correcting the FDA formula by multiplying it by arbitrary numbers lacking scientific basis," adding, "This is similar to how past scientists introduced complex orbits to explain planetary movements based on the geocentric model, which was the prevailing theory at the time."
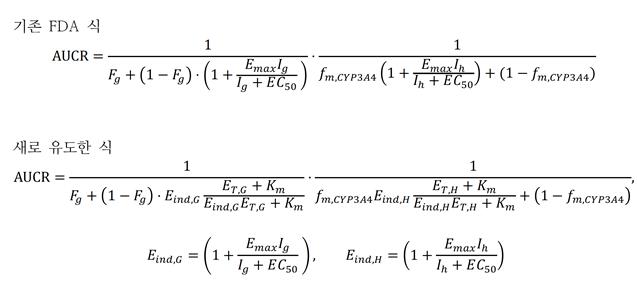 Equations presented in the FDA guidance (above) and newly derived by the research team (below) for predicting drug interactions. AUCR (area under the drug concentration-time curve) represents the ratio of change in drug concentration due to drug interactions. The research team improved the prediction accuracy of drug interactions by more than twofold compared to the existing equation. Image provided by IBS
Equations presented in the FDA guidance (above) and newly derived by the research team (below) for predicting drug interactions. AUCR (area under the drug concentration-time curve) represents the ratio of change in drug concentration due to drug interactions. The research team improved the prediction accuracy of drug interactions by more than twofold compared to the existing equation. Image provided by IBS
The research team developed a new formula to explain drug interactions through a collaborative study between mathematics and pharmacy. Instead of the previously unquestioned formula, they derived a new formula that can accurately predict drug metabolism rates regardless of enzyme concentration. They then used the newly derived formula to predict drug interactions and compared the results with experimentally measured values. The results confirmed that prediction accuracy increased more than twofold without artificial correction. While the existing FDA formula predicted drug interactions within a twofold error range 38% of the time, the revised formula achieved 80% accuracy.
Most pharmaceuticals, excluding biological products, evaluate drug interactions according to FDA guidance. These results directly affect drug efficacy and side effects. This underscores the need for drug interaction research and prescriptions using accurate formulas.
Professor Kim Sang-gyeom of Chungnam National University College of Pharmacy stated, "Improving the accuracy of drug interaction predictions will contribute to the success rate of new drug development and the efficiency of drugs in clinical practice," adding, "Since the paper was published in a top journal in the field of clinical pharmacology, we expect the FDA guidance to be revised based on these research results."
Kim Jae-kyung of IBS CI said, "Thanks to the collaborative research between mathematics and pharmacy, we were able to revise a formula that was considered unquestionably correct and find clues for a healthier life for humanity," adding, "I dream that the 'K-formula' will be included in the U.S. FDA guidance."
The research results were published online on December 15 last year in the international journal in the field of clinical pharmacology, Clinical Pharmacology and Therapeutics (IF 7.051).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















