Pig Organ Transplantation into Patients
MFDS Approves Phase 1 Clinical Trial of 'Xenogeneic Islet Transplantation' for Type 1 Diabetes Patients
Emerging Alternative to Address Organ Shortage
 Research on 'xenotransplantation,' the transplantation of animal organs into humans, is actively being conducted worldwide. Pigs are currently the most extensively studied animals in this field.
Research on 'xenotransplantation,' the transplantation of animal organs into humans, is actively being conducted worldwide. Pigs are currently the most extensively studied animals in this field. [Image source=Pixabay]
[Asia Economy Reporter Lee Gwan-joo] With the recent approval of a clinical trial for ‘xenogeneic islet transplantation’ adhering to international standards in South Korea, expectations for xenotransplantation are growing. Xenotransplantation refers to the transplantation of organs, tissues, or cells obtained from animals into humans for therapeutic purposes. Amid a global shortage of transplant organs, the establishment of xenotransplantation technology is expected to enable countless patients to gain new life through organ transplantation.
World’s First International Standard-Compliant ‘Xenogeneic Islet Transplantation’ Phase 1 Clinical Trial Approved
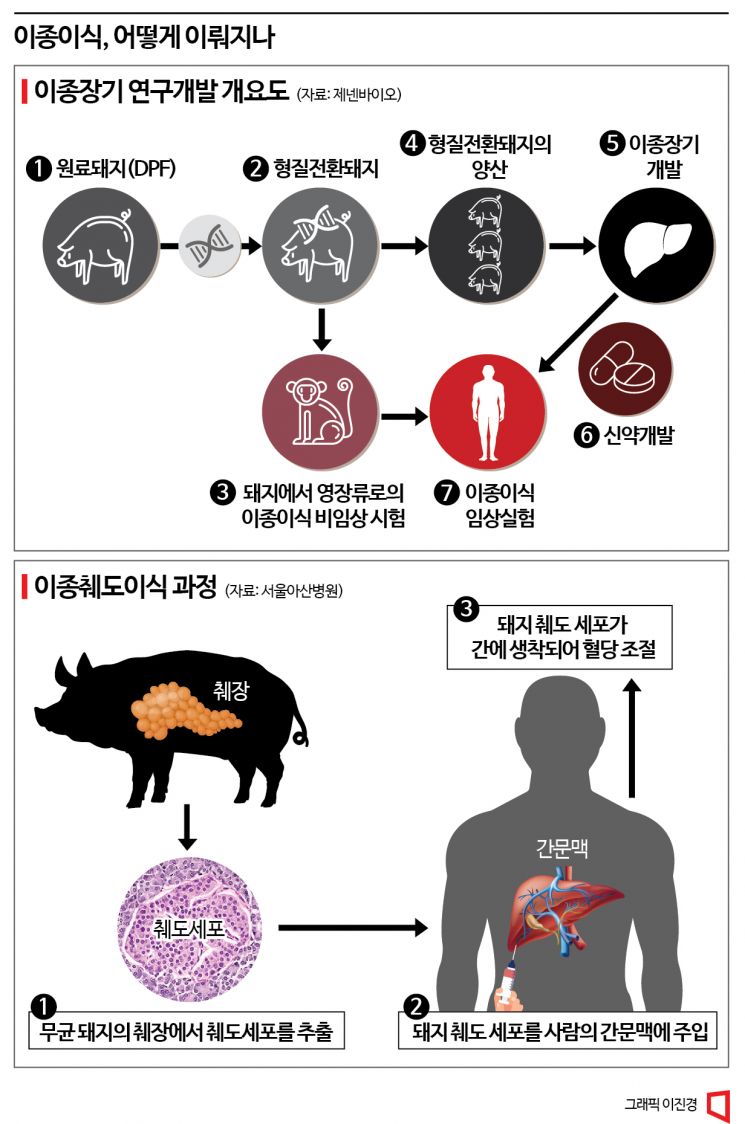
According to industry sources on the 8th, the Ministry of Food and Drug Safety approved the Phase 1 clinical trial applied for by Genenbio on the 5th to evaluate the safety and efficacy of pig islet transplantation. This Phase 1 trial involves transplanting sterile pig islets into two patients with type 1 diabetes and monitoring them for two years to confirm safety and efficacy. In prior preclinical trials, non-human primate diabetes models receiving sterile pig islet transplants showed a significant reduction in insulin requirements compared to before transplantation.
Islet transplantation is typically performed on patients with type 1 diabetes whose blood sugar cannot be controlled by insulin administration. The method involves extracting the donor’s pancreas and isolating only the insulin-secreting islet cells for transplantation. However, pancreas transplantation itself is rare, and the conditions for cell isolation are stringent, making it difficult to transplant to many patients. Xenogeneic islet transplantation using pig islets is gaining attention for these reasons. It is evaluated as having a higher potential for commercialization worldwide because the burden and risk to patients are lower than with other xenogeneic organs. In particular, it could be a fundamental treatment for patients with type 1 diabetes.
This clinical trial is being conducted jointly by Genenbio and Gachon University Gil Medical Center, with collaboration from researchers who were part of the Seoul National University Bio-Xenotransplantation Development Project Group. For this trial, Genenbio will produce a cell therapy product from sterile pig islets at the xenogeneic islet cell therapy manufacturing facility established within Gil Medical Center and supply it to the hospital. Recruitment of clinical trial participants is expected to begin in the first half of next year.
Genenbio applied for the Investigational New Drug (IND) application in August last year and received approval after submitting supplementary materials to the Ministry of Food and Drug Safety, taking one year and four months. Notably, this approval marks the world’s first xenogeneic islet transplantation clinical trial that complies with international standards set by organizations such as the World Health Organization (WHO) and the International Xenotransplantation Association (IXA). Kim Sung-joo, CEO of Genenbio and former head of the Organ Transplant Center at Samsung Seoul Hospital, stated, “Xenogeneic islet transplantation, a new treatment method in advanced regenerative medicine, has entered clinical trials after meticulous supplementation. We are committed to developing the first xenotransplantation-based pharmaceutical product and an effective treatment for diabetes through xenogeneic islet transplantation.”
Can Xenotransplantation Solve the Global Organ Shortage?
The growing attention to xenotransplantation stems from the imbalance between demand and supply. There are many patients waiting for transplants, but organ supply is limited. According to the National Organ, Tissue, and Blood Management Agency, as of the third quarter of this year, there were 48,794 patients on the waiting list for organ transplants in South Korea, while only 3,878 transplants were actually performed. Regarding islet transplantation alone, 30 patients were on the waiting list, but not a single transplant was carried out.
This phenomenon is similar worldwide, and xenotransplantation research is actively progressing in various countries. China approved clinical trials for pig corneas in 2015 and has transplanted them into humans. In the United States, in January this year, the Food and Drug Administration (FDA) granted compassionate use approval, leading to the first successful transplantation of a genetically modified pig heart into a human. Although the heart transplant patient died two months later, the case is regarded as a meaningful step demonstrating the scientific progress and potential of xenotransplantation.
One of the strengths of xenotransplantation is that, since animal organs are used, the supply source is abundant, making it easier to retry transplantation if it fails, and reducing waiting times to allow for planned surgeries. Thanks to advances in genetic engineering, it is expected that patient-customized organ production will be possible in the long term. Currently, pigs are the most studied animals. They are genetically and anatomically similar to humans in terms of organ size and shape, and are relatively free from disease infection and ethical issues. Their short gestation period and high fertility also facilitate the mass supply of organs.
However, there is still a long way to go before xenotransplantation becomes a standard clinical guideline. The Biotechnology Policy Research Center identified key challenges in its report titled ‘Xenotransplantation Clinical Trials One Step Closer,’ including ▲elimination of latent pig viruses ▲development of genetically modified pigs suitable for organ production ▲research on customized transplantation strategies. These are also the main focus areas for companies involved in xenotransplantation research. The report stated, “If the ultimate goal is transplantation into humans, various studies including clinical trials of pig organ transplantation in humans are necessary.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.