Hemgenix, a Treatment for Hemophilia B
Rare Disease Affecting 434 Patients in Korea
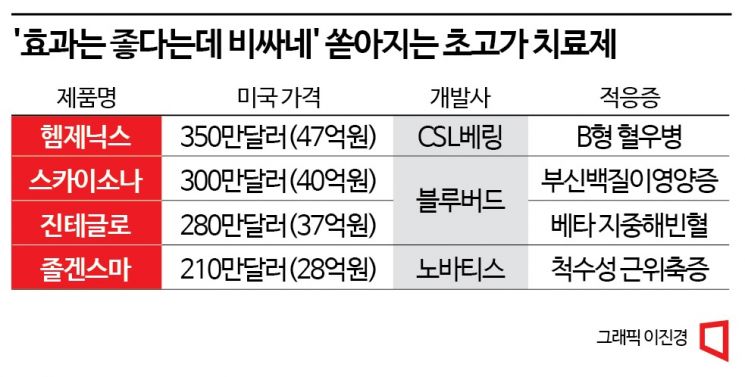
Following Novartis' Zolgensma at 2.8 Billion Won,
Jinteglo at 3.7 Billion and Skysona at 4 Billion Won
Ultra-High-Cost Treatments Flood the Market One After Another
[Asia Economy Reporter Lee Chun-hee] Zolgensma, Zynteglo, Skysona. These drugs are classified as 'ultra-high-cost therapies' that cost at least 2 billion KRW per single dose. Each costs 2.8 billion KRW, 3.7 billion KRW, and 4 billion KRW respectively based on U.S. prices. This time, a drug with a single dose cost of as much as 4.7 billion KRW has emerged.
According to the related industry on the 28th, the U.S. Food and Drug Administration (FDA) recently approved CSL Behring's hemophilia B treatment 'Hemgenix.' It is the first 'one-shot' gene therapy that can cure the rare disease hemophilia B with a single administration. Hemophilia B is a congenital bleeding disorder caused by a single gene defect, resulting from a deficiency of coagulation factor IX, which is mainly produced in the liver. Until now, treatment involved preventive injections of the deficient coagulation factor IX to temporarily block the deficiency, requiring lifelong administration.
However, according to clinical results disclosed by CSL Behring, an average of 36.7% to 36.9% of the treated group showed endogenous coagulation factor IX activity. The annual bleeding rate (ARR) of patients decreased by 54% compared to before the injection, and 94% discontinued preventive coagulation factor injection therapy.
Hemophilia, broadly classified into types A, B, and C, is a congenital disease caused by a deficiency of coagulation factors. Type A involves factor VIII deficiency, type B involves factor IX deficiency, and type C involves factor XI deficiency. About 80% of patients have type A, and about 20% have type B. Type C is very rare. In South Korea, as of 2020, there were reportedly 1,746 patients with type A and 434 with type B, totaling 2,509 patients.
Considering that there has been no fundamental cure for hemophilia B as well as type A until now, the approval of Hemgenix draws attention as the development of the first drug capable of curing hemophilia. Previously, type A treatments included Roche's 'Hemlibra,' Takeda's 'Advate' and 'Adynovate,' and SK Chemicals-CSL Behring's 'Apsila,' while type B treatments included Pfizer's 'Benefix' and Sanofi's 'Alprolix.' However, all these therapies supplemented the deficient coagulation factors and did not achieve a fundamental cure.
However, concerns have been raised that the high price of Hemgenix could impose a heavy burden on patients. The single-dose price of Hemgenix reaches as high as 3.5 million USD (approximately 4.7 billion KRW). Even if it achieves near-curative effects with a single dose, if insurance coverage is not applied, the price may make it difficult to administer easily.
Starting with Novartis's leukemia treatment 'Kymriah' and spinal muscular atrophy (SMA) treatment 'Zolgensma,' the development of cell and gene therapies (CGT), including chimeric antigen receptor (CAR)-T cell therapies, has accelerated, leading to the emergence of such ultra-high-cost therapies one after another.
In August, Bluebird Bio's transfusion-dependent beta-thalassemia treatment 'Zynteglo' received FDA approval, surpassing Zolgensma's U.S. price record of 2.125 million USD (approximately 2.8 billion KRW) with a price of 2.8 million USD (approximately 3.7 billion KRW) at once. Then, the following month, Bluebird's adrenoleukodystrophy (CALD) treatment 'Skysona,' also known as 'Lorenzo's oil disease,' broke the record again with a price of 3 million USD (approximately 4 billion KRW).
If these ultra-high-cost therapies enter the domestic market, the issue of whether they will be covered by the National Health Insurance is expected to become a key point, given that as 'one-shot' therapies, they can treat rare and intractable diseases with a single administration. In fact, for Zolgensma, the domestic price was set at 1.96 billion KRW, but through rare disease special calculation exceptions and out-of-pocket maximum payment systems, the patient's burden has been reduced to a maximum of about 6 million KRW.
In this regard, the government and the National Health Insurance Service are continuously promoting risk-sharing agreements with pharmaceutical companies, considering actual drug usage and effectiveness. If the number of patients exceeds a pre-set limit, or if the disease progresses or prognosis does not improve after treatment, the pharmaceutical company bears the full cost of treatment or refunds part of the cost to the National Health Insurance Service.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![A Woman Who Jumps Holding a Stolen Dior Bag... The Mind-Shaking, Bizarre Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















