[Asia Economy Reporter Chunhee Lee] Kangstem Biotech announced on the 16th that it received approval from the Ministry of Food and Drug Safety the day before for the clinical trial plan (IND) for the phase 1 and 2a clinical trials of its stem cell-based combination formulation osteoarthritis treatment, ‘Purestem-OA Kit Injection’.
Purestem-OA Kit Injection is a combination formulation that administers umbilical cord blood-derived mesenchymal stem cells together with acellular cartilage matrix, aiming to be a disease-modifying osteoarthritis drug (DMOAD). In particular, it is explained that a single injection into the knee joint cavity without surgery allows the administered cells to differentiate into chondrocytes, directly contributing to cartilage tissue regeneration, thus expecting a fundamental therapeutic effect.
This clinical trial will be conducted on patients with moderate osteoarthritis corresponding to grades 3 to 4 of the international standard for cartilage defect severity, the 'ICRS (International Cartilage Repair Society)'. After a single injection of Purestem-OA Kit Injection into the knee joint cavity, the trial will explore safety assurance and efficacy in structural improvement including cartilage regeneration and pain management.
In phase 1, tolerability and safety of low, medium, and high doses will be confirmed in up to 18 osteoarthritis patients. Subsequently, phase 2a will compare the efficacy over 6 months of two dose groups confirmed safe with a placebo group in a total of 50 osteoarthritis patients. Linked with long-term follow-up, efficacy at the 12-month point will also be explored.
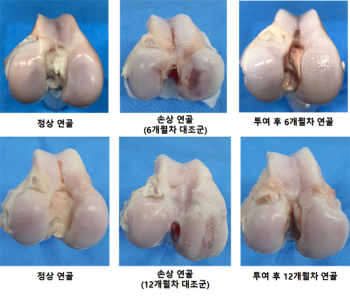 Gangstem Biotech explained that after administering 'Purestem-OA Kit' to goats induced with osteoarthritis, they evaluated the structural improvement of the joint surface at 6 and 12 months, confirming that the structure improved to a level almost equivalent to normal cartilage (regeneration of cartilage tissue and meniscus area, inflammation suppression). (Photo by Gangstem Biotech)
Gangstem Biotech explained that after administering 'Purestem-OA Kit' to goats induced with osteoarthritis, they evaluated the structural improvement of the joint surface at 6 and 12 months, confirming that the structure improved to a level almost equivalent to normal cartilage (regeneration of cartilage tissue and meniscus area, inflammation suppression). (Photo by Gangstem Biotech)
Kangstem Biotech explained that in animal tests using goats as a model, results such as inflammation suppression and regeneration of cartilage tissue and meniscus areas were observed, and it was confirmed that the administered cells remained in the regenerated tissue for up to 6 months. Regarding the use of goats in preclinical studies, it was stated that their weight of about 45 to 50 kg is relatively similar to that of humans, and since they use their knees extensively, they are appropriate for inducing osteoarthritis and evaluating efficacy.
Jongcheon Na, CEO of Kangstem Biotech, said, “There is concentrated interest from overseas partners in this clinical trial,” adding, “Preparations for global commercialization and technology transfer are also underway.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

















