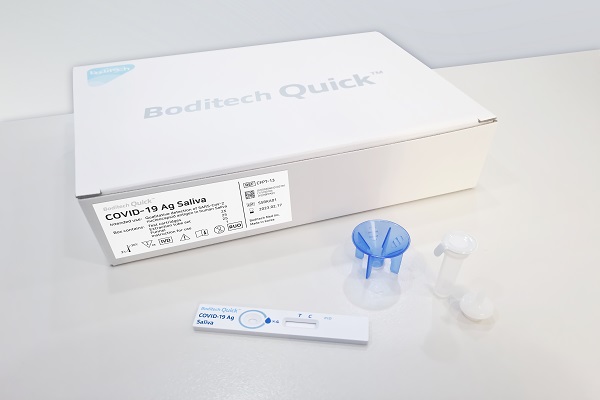
[Asia Economy Reporter Lee Gwan-joo] Boditech Med announced on the 4th that it has obtained domestic approval from the Ministry of Food and Drug Safety for the saliva self-diagnostic kit 'Boditech Quick COVID-19 Ag Saliva,' which can determine COVID-19 infection within 15 minutes using saliva.
In clinical trials conducted on 281 subjects, the product showed a sensitivity and specificity of 93.8% and 99.5%, respectively. The sensitivity of 93.8% is the highest among saliva diagnostic kits approved domestically.
The method involves coughing up saliva from the throat and spitting it into the solution tube up to the marked line, then mixing it with the buffer inside the solution tube and dropping it onto the test cartridge. The test results can be confirmed within 15 minutes.
It also has the capability to detect COVID-19 variants. In variant detection experiments, it was confirmed that all variants identified so far, including the Omicron subvariant BA.5, can be detected.
Choi Eui-yeol, CEO of Boditech Med, said, “The saliva diagnostic kit approved this time has the best performance among the products released so far,” adding, “It can detect all confirmed variants to date, making it a very valuable diagnostic kit not only for primary medical institutions and emergency rooms but also for the elderly and children.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.