Celltrion Signs Agreement with Pinobio, Holder of ADC Platform
Based on 'Click Chemistry' Awarded Nobel Prize in Chemistry This Year
Antibody-Drug Conjugates Deliver Toxins Specifically to Cancer Cells
'Enhertu' Shows Innovative Results, Emerging Prominently
Fierce Competition to Secure Technology Amid Rapid Market Growth
Domestic Companies Like Legochem and Pinobio Also Participate
[Asia Economy Reporter Lee Chun-hee] Interest is growing in antibody-drug conjugate (ADC) pharmaceuticals, known as 'cruise missiles' that bombard cancer with drugs. As innovative results surpassing existing treatments emerge, competition among companies to develop the technology is intensifying.
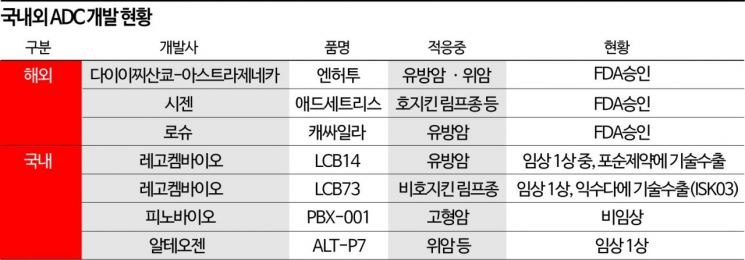
According to industry sources on the 19th, Celltrion recently signed a contract to acquire an option to use ADC platform technology from domestic biotech company Pinobio. Since 2019, Celltrion has been actively securing ADC technology by collaborating consecutively with companies possessing ADC technology such as iProgen and last year, ExsuDa Therapeutics. Using Pinobio's platform 'Pino-ADC,' they are pushing forward the development of ADC anticancer drugs targeting solid tumors. They have secured technology use options for a total of 15 targets. If all options are exercised, the total contract value could increase up to $1.2428 billion (approximately 1.7758 trillion KRW).
As the name suggests, ADCs are pharmaceuticals that conjugate antibodies and drugs. By linking antibodies that bind to cancer antigens with cytotoxic drugs (payloads) that can kill cancer cells via a linker, they can effectively deliver toxins only to cancer cells like missiles, making them a next-generation anticancer technology. This year, the selection of Professor Carolyn Bertozzi of Stanford University, USA, as a Nobel Chemistry laureate is closely related to ADCs. They were recognized for inventing 'click chemistry.' The application of click chemistry to organisms is called 'bioorthogonal chemistry.' When different functional groups meet, they do not interact with other proteins or organics but only with their designated partners. ADCs were developed based on this principle.
In particular, the ADC 'Enhertu,' jointly developed by Daiichi Sankyo and AstraZeneca, demonstrated innovative results targeting HER2-low expression patients, who account for half of metastatic breast cancer patients, and received standing ovations at the American Society of Clinical Oncology (ASCO) in June. As innovative results continue to appear, market attention is significantly increasing.
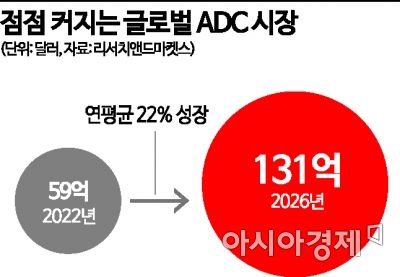
Currently, starting with Seagen's lymphoma treatment 'Adcetris' approved by the U.S. Food and Drug Administration (FDA) in 2011, a total of 12 drugs including Enhertu have received FDA approval. Among them, Adcetris and Roche's 'Kadcyla' grew into blockbuster drugs with sales of $1.938 billion (approximately 2.7607 trillion KRW) and $2.169 billion (approximately 3 trillion KRW) respectively as of last year. According to market research firm ResearchAndMarkets, the global ADC pharmaceutical market is expected to grow from $5.9 billion (approximately 8.4075 trillion KRW) this year at an average annual growth rate of 22%, reaching $13.1 billion (approximately 18.6675 trillion KRW) by 2026. Enhertu, which has been launched in the market after receiving approvals from the FDA and Korea's Ministry of Food and Drug Safety, is expected to generate sales of $7.741 billion (approximately 11 trillion KRW) by 2028.
Domestically, companies such as LegoChem Biosciences and Pinobio are also developing ADC technology. Using LegoChem Biosciences' 'ConjuALL' platform, they are developing 'LCB14' and 'LCB73.' LCB14 showed a significant objective response rate (ORR) of 67% in phase 1 trials conducted by Chinese partner Fosun Pharma, and LCB73 is undergoing phase 1 clinical trials after technology transfer to ExsuDa.
Pinobio is preparing a platform that overcomes toxicity, a risk factor of ADCs. In fact, Pfizer's ADC 'Mylotarg,' which was the first ADC approved by the FDA in 2010 before Adcetris, had its approval withdrawn due to toxicity issues. Since ADCs induce cancer cell death through cytotoxic efficacy, if the payload detaches before reaching cancer cells, hematologic toxic side effects may occur.
Pinobio developed the 'Pino-ADC' platform, which uses camptothecin-class inhibitory payloads with potential to overcome safety and resistance issues, thereby enhancing safety. Through this, they are independently developing the Trop-2 targeting ADC anticancer drug 'PBX-001.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.