[Asia Economy Reporter Jo In-kyung] The Ministry of Food and Drug Safety announced on the 14th that it has granted national batch release approval for 780,000 doses of Pfizer's COVID-19 bivalent vaccine 'Comirnaty 2-dose,' developed to respond to the Omicron variant (BA.1).
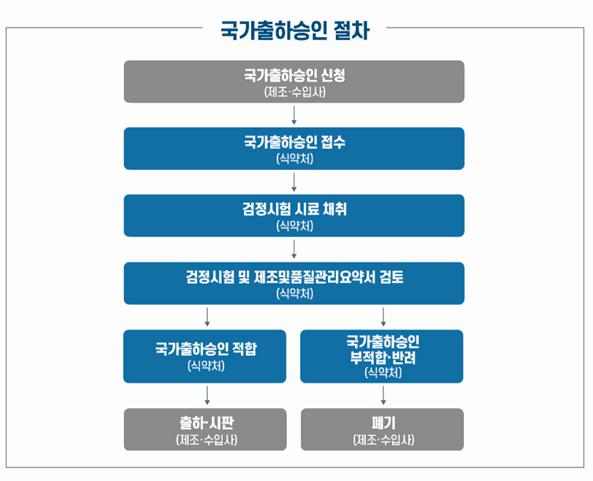
National batch release approval is a system in which the government comprehensively evaluates the results of national inspection tests and the manufacturer's production and testing results before the vaccine is distributed in the market, confirming the quality once again.
Comirnaty 2-dose is a vaccine developed to respond to both the original COVID-19 virus and the Omicron variant virus, and this approval was granted for imported products authorized on the 7th.
The Ministry of Food and Drug Safety judged that Comirnaty 2-dose meets the approved quality standards after conducting an evaluation.
The Ministry stated, "With the national batch release approval of the COVID-19 bivalent vaccine, it is expected to help prevent COVID-19."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.