Emergence of Migraine Treatments with Various Mechanisms
'Emgality', 'Ajovy', and 'Reyvow'
Botulinum Toxin, an Existing Treatment, Also Competes
Coverage Hindered by Concerns Over Misuse
[Asia Economy Reporter Lee Chun-hee] As new migraine treatments with novel mechanisms are being introduced domestically one after another, the migraine treatment market is attracting attention. Competition within the existing major treatment method, botulinum toxin (BTX) market, is also intensifying.
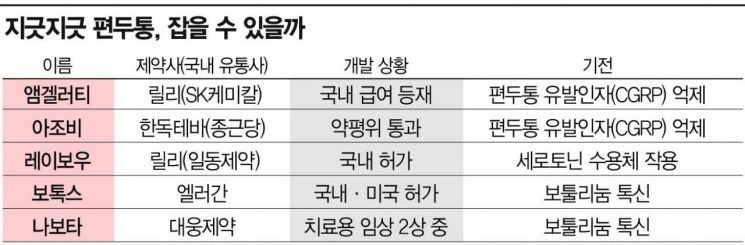
According to the pharmaceutical and bio industry on the 4th, Lilly's migraine preventive treatment 'Emgality' (active ingredient galcanezumab) has been included in the National Health Insurance coverage since last month. As a result, the annual patient cost burden, which was 9.1 million KRW without insurance coverage, has significantly decreased to about 1.15 million KRW. Clinical trials showed that the monthly number of migraine days decreased by an average of about 4 days during the 6-month treatment period. It prevents and treats migraines by inhibiting calcitonin gene-related peptide (CGRP), a major substance known to cause migraine symptoms in the brain.
Migraine, characterized by periodic pain attacks caused by dysfunction of the blood vessels in the head, is one of the most common diseases. If pain occurs 15 days or more per month, it is classified as Chronic Migraine; if less than 15 days, it is Episodic Migraine. It is estimated that about 1 billion people worldwide suffer from migraines. Market research firm GlobalData estimated that the global migraine treatment market has grown at an average annual rate of 3.7% since 2020 and will reach 5.1 billion USD (approximately 7.2772 trillion KRW) by 2024.
 Lilly's migraine preventive treatment 'Emgality' and Handok Teva's migraine preventive treatment 'Ajovy' (from left).
Lilly's migraine preventive treatment 'Emgality' and Handok Teva's migraine preventive treatment 'Ajovy' (from left).
Hadong Teva's 'Ajovy', which also has a CGRP inhibition mechanism like Emgality, is accelerating its entry into insurance coverage. Ajovy received approval for insurance coverage appropriateness from the Health Insurance Review and Assessment Service's Drug Benefit Evaluation Committee last month, signaling a green light for coverage. Clinical trials showed that the monthly number of migraine days in the treatment group decreased by about 4 days.
With the insurance coverage of these two drugs following one another, market competition between SK Chemicals, which distributes Emgality domestically, and Chong Kun Dang, which handles Ajovy, is expected to intensify. However, since Emgality is administered twice a month and Ajovy is administered once a month or once every three months, and because the effectiveness of migraine drugs varies from patient to patient, it is predicted that it will be difficult for either side to easily gain dominance.
Il Dong Pharmaceutical is preparing to launch Lilly's acute migraine treatment 'Reyvow', for which it purchased the rights from the development stage in 2013. It acts on the serotonin (5-HT) 1F receptor to rapidly relieve acute migraine symptoms regardless of the presence of prodromal symptoms. Clinical trials showed that the pain relief rate within 2 hours was about four times higher compared to the placebo group. However, after undergoing a review by the Drug Benefit Evaluation Committee in July with the conclusion 'insurance coverage appropriateness if accepted below the evaluated price,' the company has started to reconsider its launch strategy. Since the price suggested by the committee is low, it is reported that they are considering options such as re-pursuing insurance listing through additional negotiations or launching first without insurance coverage and then aiming for expanded coverage.
The treatment BTX market, which has been used as one of the major migraine treatment methods, is also becoming more competitive. Allergan's 'Botox' has been used since it was approved by the FDA in 2010 as a treatment for chronic migraine. The principle is to inject BTX into nerve distribution points from the forehead to the shoulders to stabilize the nerves surrounding the brain.
In Korea, BTX developers are also developing treatments for migraines as they seek to secure treatment indications. Daewoong Pharmaceutical is conducting a Phase 2 clinical trial for 'Nabota' targeting chronic and episodic migraines. The top-line results are expected to be announced in the second half of next year. Notably, the indication for episodic migraine is the first among BTX products to be researched.
However, insurance coverage for BTX for migraines has not been implemented. Although there have been continuous demands for coverage, discussions have stalled because there is a high risk of misuse for cosmetic purposes if coverage is granted.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.