FDA Approves 'Daxxify' with Average 6-Month Interval
Previously, Botulinum Required Every 3-4 Months
Developers Expand Indications Amid Therapeutic Market Growth
Daewoong's 'Nabota' Conducts Clinical Trials for Neck Muscle Disorders and Headaches
Hugel and Medytox Also Research Various Indications
[Asia Economy Reporter Lee Chun-hee] The competition in the botulinum toxin (BTX) market is intensifying as 'Daxxify,' which has a duration of effect more than twice that of existing BTXs, has been approved in the United States, and BTX developers are rushing to expand therapeutic indications.
According to the industry on the 30th, on the 8th (local time), the U.S. Food and Drug Administration (FDA) approved Daxxify, a BTX from Revance Therapeutics. While conventional BTX requires injections every 3 to 4 months, Revance reported that in Phase 3 clinical trials, Daxxify showed wrinkle improvement effects lasting an average of 6 months and up to 9 months. With greatly improved dosing convenience, it is being evaluated overseas as a game-changer that could significantly shake up the BTX market.
However, some view this with skepticism. A BTX company official said, "Our own analysis suggests that the prolonged effect of Daxxify is likely due to increased dosage," adding, "Internally, we believe there is not a high need for additional research and development (R&D) yet." Another company official said, "Existing products can also maintain effects for 6 to 12 months depending on the patient," and "We need to observe Daxxify's duration a bit longer."
Botulinum Toxin, Originally 'For Therapeutic Use'
Accordingly, domestic pharmaceutical companies are recently focusing on expanding therapeutic indications. In fact, BTX was first used therapeutically for conditions such as eyelid spasms before its wrinkle-improving effects were confirmed, leading to cosmetic use as well. Especially, although 90% of the domestic BTX market is cosmetic, according to Daedal Research, the global BTX market size is $5.9 billion (approximately 8.4488 trillion KRW), with the therapeutic market at $3.2 billion (approximately 4.5824 trillion KRW), representing a larger share. With continuous growth expected in the therapeutic market and the domestic market not yet fully opened, there is great potential. Accordingly, Daewoong Pharmaceutical, Hugel, and Medytox, domestic BTX developers, are also working to secure various therapeutic indications.
Daewoong Pharmaceutical was the first domestic BTX company to enter the overseas therapeutic market. Its U.S. partner Ion Biopharma recently announced topline results from a Phase 2 clinical trial of Daewoong's 'Nabota' for cervical dystonia in the U.S. Cervical dystonia is a condition where neck muscles contract or spasm involuntarily, causing abnormal neck rotation. In this trial, all low, medium, and high dose groups of Nabota showed significant improvement compared to placebo in the primary endpoint, the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS).
Ion Biopharma is also conducting a Phase 2 clinical trial for chronic and episodic migraine. Notably, episodic migraine is an indication that no existing BTX product has secured. Nabota also holds therapeutic indications domestically for post-stroke upper limb spasticity, eyelid spasms, and benign masseter hypertrophy (square jaw).
Hugel's 'Botulax' has secured indications for eyelid spasms, post-stroke upper limb muscle spasticity, and equinus foot deformity due to spasticity in pediatric cerebral palsy patients. Additionally, clinical trials are underway for square jaw, cervical dystonia, and overactive bladder.
Medytox's 'Medytoxine' has indications for cervical dystonia, post-stroke upper limb muscle spasticity, equinus foot deformity in pediatric cerebral palsy patients, and eyelid spasms. Research is ongoing to add indications such as migraine and hyperhidrosis.
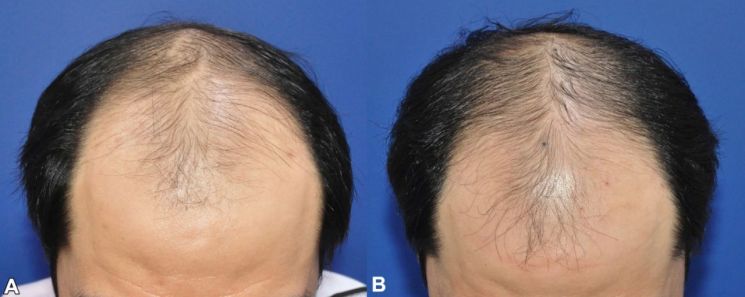 Researcher's Visual Assessment Before and After Botulinum Toxin 'Nabota' Injection in Male Pattern Baldness Areas (Photo by Daewoong Pharmaceutical)
Researcher's Visual Assessment Before and After Botulinum Toxin 'Nabota' Injection in Male Pattern Baldness Areas (Photo by Daewoong Pharmaceutical)
The possibility of hair loss treatment using BTX is also being researched. Daewoong Pharmaceutical announced research results showing that direct administration of Nabota to the scalp of male pattern hair loss patients statistically significantly increased hair count at 24 weeks. In the U.S., Allergan is conducting a Phase 2 clinical trial to confirm the efficacy of BTX 'Xeomin' for androgenetic alopecia treatment.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![A Woman with 50 Million Won Debt Clutches a Stolen Dior Bag and Jumps... A Monster Is Born [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














